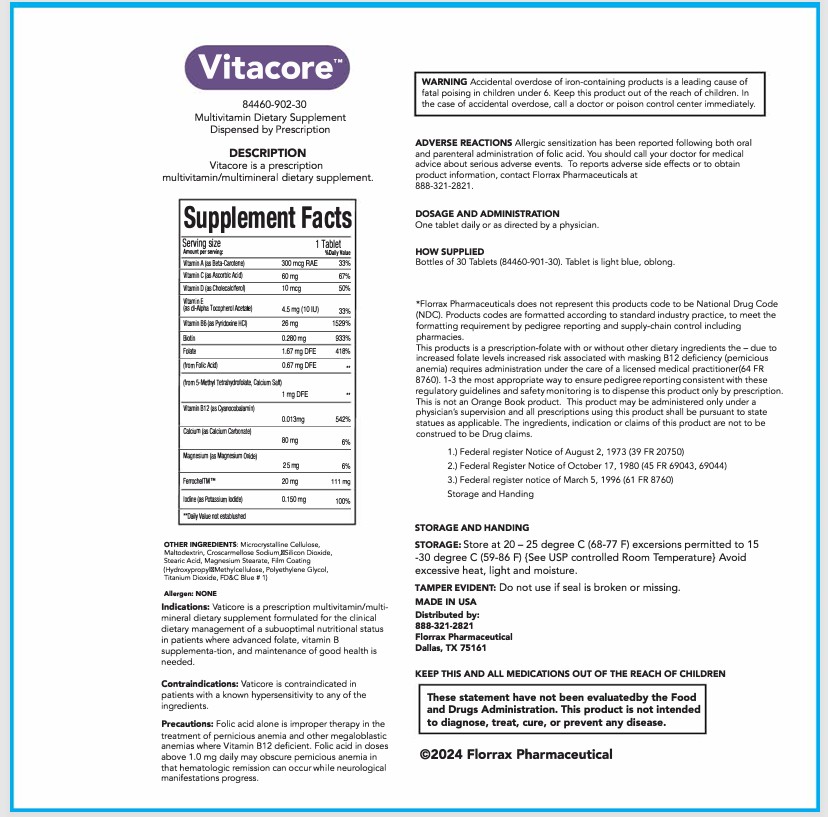
VITACORE- multivitamin tablet
Vitacore by
Drug Labeling and Warnings
Vitacore by is a Prescription medication manufactured, distributed, or labeled by Florrax Pharmaceutical Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- DESCRIPTION
- INDICATIONS & USAGE
- CONTRAINDICATIONS
- PRECAUTIONS
- WARNINGS
- ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
-
SPL UNCLASSIFIED SECTION
*Florrax Pharmaceuticals does not represent this products code to be National Drug Code
(NDC). Products codes are formatted according to standard industry practice, to meet the
formatting requirement by pedigree reporting and supply-chain control including
pharmacies.
This products is a prescription-folate with or without other dietary ingredients the - due to
increased folate levels increased risk associated with masking B12 deficiency (pernicious
anemia) requires administration under the care of a licensed medical practitioner(64 FR
8760). 1-3 the most appropriate way to ensure pedigree reporting consistent with these
regulatory guidelines and safety monitoring is to dispense this product only by prescription.
This is not an Orange Book product. This product may be administered only under a
physician's supervision and all prescriptions using this product shall be pursuant to state
statues as applicable. The ingredients, indication or claims of this product are not to be
construed to be Drug claims.
1.) Federal register Notice of August 2, 1973 (39 FR 20750)
2.) Federal Register Notice of October 17, 1980 (45 FR 69043, 69044)
3.) Federal register notice of March 5, 1996 (61 FR 8760)
Storage and Handing -
STORAGE AND HANDLING
STORAGE: Store at 20- 25 degree C (68-77 F) excersions permitted to 15-30 degree C (59-86 F) {See USP controlled Room Temperature} Avoid excessive heat, light and moisture.
TAMPER EVIDENT: Do not use if seal is broken or missing.
MADE IN USA
Distributed by:
888-321-2821
Florrax Pharmaceutical
Dallas, TX 75161 - OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VITACORE
multivitamin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 84460-902 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERROUS BISGLYCINATE (UNII: SFW1D987QV) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 20 mg POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) POTASSIUM IODIDE 0.15 mg BETA CAROTENE (UNII: 01YAE03M7J) (BETA CAROTENE - UNII:01YAE03M7J) BETA CAROTENE 0.3 mg ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 60 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 0.1 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 26 mg BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 0.28 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1.67 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 0.013 mg CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 80 mg MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CATION 25 mg .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 4.5 mg Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) Product Characteristics Color blue Score no score Shape OVAL Size 12mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 84460-902-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/24/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/24/2025 Labeler - Florrax Pharmaceutical Corp. (119257612)
Trademark Results [Vitacore]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VITACORE 90132677 not registered Live/Pending |
Bassam Idris 2020-08-24 |
 VITACORE 87555591 not registered Dead/Abandoned |
Fit Foods Ltd. 2017-08-03 |
 VITACORE 87513025 not registered Dead/Abandoned |
Vitacore 2017-06-30 |
 VITACORE 86420441 not registered Dead/Abandoned |
GTI USA, LLC 2014-10-10 |
 VITACORE 86179977 4718968 Live/Registered |
"Durable" Hunke & Jochheim GmbH & Co. Kommanditgesellschaft 2014-01-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.