VISUDYNE- verteporfin for injection injection, powder, lyophilized, for solution
Visudyne by
Drug Labeling and Warnings
Visudyne by is a Prescription medication manufactured, distributed, or labeled by Bausch & Lomb Incorporated, Alcami Carolinas Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VISUDYNE safely and effectively. See full prescribing information for VISUDYNE.
VISUDYNE® (verteporfin for injection), for intravenous use
Initial U.S. Approval: 2000INDICATIONS AND USAGE
VISUDYNE (verteporfin for injection) therapy is a photoenhancer indicated for the treatment of patients with predominantly classic subfoveal choroidal neovascularization due to age-related macular degeneration, pathologic myopia or presumed ocular histoplasmosis. (1)
DOSAGE AND ADMINISTRATION
- Recommended Dose: 6 mg/m2 body surface area. (2.2)
- Reconstitution: Reconstitute each vial of VISUDYNE with 7 mL of Sterile Water for Injection to provide 7.5 mL containing 2 mg/mL of verteporfin. Reconstituted VISUDYNE must be protected from light and used within 4 hours. (2.3)
- Dilution: Dilute desired dose of reconstituted VISUDYNE with 5% Dextrose for Injection to a total infusion volume of 30 mL. (2.3)
- Infusion: Administer intravenously over 10 minutes at a rate of 3 mL/minute, using an appropriate syringe pump and in-line filter. (2.3)
- Light Administration: The recommended light dose is 50 J/cm2 of neovascular lesion administered at an intensity of 600 mW/cm2. The wavelength of the laser light should be 689±3 nm. This light dose is administered over 83 seconds, starting 15 minutes after the start of the VISUDYNE infusion. (2.4)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
VISUDYNE (verteporfin for injection) is contraindicated for patients with porphyria or a known hypersensitivity to any component of this preparation. (4)
WARNINGS AND PRECAUTIONS
- Extravasation: If extravasation occurs, the infusion should be stopped immediately. The extravasation area must be thoroughly protected from direct light until swelling and discoloration have faded in order to prevent the occurrence of local burn. (5.1)
- Exposure to Sun or Direct Light: Following injection with VISUDYNE (verteporfin for injection), care should be taken to avoid exposure of skin or eyes to direct sunlight or bright indoor light for 5 days. (5.2)
- Anaphylactic Reactions: Immediately discontinue administration of VISUDYNE and initiate appropriate therapy if an anaphylactic or other serious allergic reaction occurs during or following infusion. (5.4)
ADVERSE REACTIONS
Most common adverse reactions (incidence ˃10%) are injection site reactions and visual disturbances. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-553-5340 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage of VISUDYNE
2.3 VISUDYNE Administration
2.4 Light Administration
2.5 Concurrent Bilateral Treatment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Local Adverse Reactions - Extravasation
5.2 Exposure to Sun or Direct Light
5.3 Decreased Vision After Treatment
5.4 Anaphylactic Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Age-Related Macular Degeneration
14.2 Pathologic Myopia
14.3 Presumed Ocular Histoplasmosis
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 Spills and Disposal
16.2 Accidental Exposure
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
VISUDYNE® (verteporfin for injection) therapy is indicated for the treatment of patients with predominantly classic subfoveal choroidal neovascularization (CNV) due to age-related macular degeneration (AMD), pathologic myopia or presumed ocular histoplasmosis.
There is insufficient evidence to indicate VISUDYNE for the treatment of predominantly occult subfoveal CNV.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
A course of VISUDYNE (verteporfin for injection) therapy is a two-step process requiring administration of both drug and light.
- The first step is the administration of VISUDYNE.
- The second step is the activation of VISUDYNE with light from a nonthermal diode laser.
The physician should re-evaluate the patient 3 months after treatment and if choroidal neovascular leakage is detected on fluorescein angiography, therapy may be repeated.
Lesion Size Determination
The greatest linear dimension (GLD) of the lesion should be estimated by fluorescein angiography and color fundus photography. All classic and occult CNV, blood and/or blocked fluorescence, and any serous detachments of the retinal pigment epithelium should be included for this measurement. Fundus cameras with magnification within the range of 2.4-2.6X are recommended. The GLD of the lesion on the fluorescein angiogram must be corrected for the magnification of the fundus camera to obtain the GLD of the lesion on the retina.
Spot Size Determination
The treatment spot size should be 1,000 microns larger than the GLD of the lesion on the retina to allow a 500 micron border, ensuring full coverage of the lesion. The maximum spot size used in the clinical trials was 6,400 microns. The nasal edge of the treatment spot must be positioned at least 200 microns from the temporal edge of the optic disc, even if this will result in lack of photoactivation of CNV within 200 microns of the optic nerve.
2.3 VISUDYNE Administration
Avoid contact with the eyes and skin during preparation and administration of VISUDYNE. Because of the potential to induce photosensitivity reactions, any exposed person must be protected from bright light [see Warnings and Precautions (5.1) and How Supplied/Storage and Handling (16)].
Reconstitution
Reconstitute each vial of VISUDYNE with 7 mL of Sterile Water for Injection. Each reconstituted vial provides 7.5 mL solution containing 2 mg/mL of verteporfin. Reconstituted VISUDYNE must be protected from light and used within 4 hours. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Reconstituted VISUDYNE is an opaque dark green solution.
Dilution
VISUDYNE may precipitate in saline solutions. Do not use normal saline or other parenteral solutions, except 5% Dextrose for Injection, for dilution of the reconstituted VISUDYNE. Do not mix VISUDYNE in the same solution with other drugs.
The volume of reconstituted VISUDYNE required to achieve the desired dose of 6 mg/m2 body surface area is withdrawn from the vial and diluted with 5% Dextrose for Injection to a total infusion volume of 30 mL. After dilution, protect from light and use within 4 hours.
Intravenous Infusion
The full infusion volume is administered intravenously over 10 minutes at a rate of 3 mL/minute, using an appropriate syringe pump and in-line filter. The clinical studies were conducted using a standard infusion line filter of 1.2 microns. Precautions should be taken to prevent extravasation at the injection site. If extravasation occurs, protect the site from light [see Warnings and Precautions (5.1)].
2.4 Light Administration
Initiate 689 nm wavelength laser light delivery to the patient 15 minutes after the start of the 10-minute infusion with VISUDYNE.
Photoactivation of VISUDYNE is controlled by the total light dose delivered. In the treatment of CNV, the recommended light dose is 50 J/cm2 of neovascular lesion administered at an intensity of 600 mW/cm2. This dose is administered over 83 seconds.
Light dose, light intensity, ophthalmic lens magnification factor and zoom lens setting are important parameters for the appropriate delivery of light to the predetermined treatment spot. Follow the laser system manuals for procedure set up and operations.
The laser system must deliver a stable power output at a wavelength of 689±3 nm. Light is delivered to the retina as a single circular spot via a fiber optic and a slit lamp, using a suitable ophthalmic magnification lens.
The following laser systems have been tested for compatibility with VISUDYNE and are approved for delivery of a stable power output at a wavelength of 689±3 nm:
- Coherent Opal Photoactivator laser console and modified Coherent LaserLink adapter, manufactured by Lumenis, Inc., 2400 Condensa Street, Santa Clara, CA 95051-0901,
- Zeiss VISULAS 690s laser and VISULINK PDT adapter manufactured by Carl Zeiss Meditec Inc., 5160 Hacienda Drive, Dublin, CA 94568,
- Ceralas I laser system and Ceralink Slit Lamp Adapter manufactured by Biolitec Inc., 515 Shaker Road, East Longmeadow, MA 01028,
- Quantel Activis laser console and the ZSL30 ACT, ZSL120 ACT and HSBMBQ ACT slit lamp adapters distributed by Quantel Medical, 601 Haggerty Lane, Bozeman, MT 59715.
2.5 Concurrent Bilateral Treatment
The controlled trials only allowed treatment of one eye per patient. In patients who present with eligible lesions in both eyes, physicians should evaluate the potential benefits and risks of treating both eyes concurrently. If the patient has already received previous VISUDYNE therapy in one eye with an acceptable safety profile, both eyes can be treated concurrently after a single administration of VISUDYNE. The more aggressive lesion should be treated first, at 15 minutes after the start of infusion. Immediately at the end of light application to the first eye, the laser settings should be adjusted to introduce the treatment parameters for the second eye, with the same light dose and intensity as for the first eye, starting no later than 20 minutes from the start of infusion.
In patients who present for the first time with eligible lesions in both eyes without prior VISUDYNE therapy, it is prudent to treat only one eye (the most aggressive lesion) at the first course. One week after the first course, if no significant safety issues are identified, the second eye can be treated using the same treatment regimen after a second VISUDYNE infusion. Approximately 3 months later, both eyes can be evaluated and concurrent treatment following a new VISUDYNE infusion can be started if both lesions still show evidence of leakage.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
VISUDYNE (verteporfin for injection) is contraindicated for patients with porphyria or a known hypersensitivity to any component of this preparation [see Adverse Reactions (6)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Local Adverse Reactions - Extravasation
Standard precautions should be taken during infusion of VISUDYNE (verteporfin for injection) to avoid extravasation. Examples of standard precautions include, but are not limited to:
- A free-flowing intravenous (IV) line should be established before starting VISUDYNE infusion and the line should be carefully monitored.
- Due to the possible fragility of vein walls of some elderly patients, it is strongly recommended that the largest arm vein possible, preferably antecubital, be used for injection.
- Small veins in the back of the hand should be avoided.
Extravasation of VISUDYNE, especially if the affected area is exposed to light, can cause severe pain, inflammation, swelling or discoloration at the injection site. Localized (skin) necrosis at the injection site following extravasation has
also been reported.
If extravasation does occur, the infusion should be stopped immediately. The extravasation area must be thoroughly protected from direct light until swelling and discoloration have faded in order to prevent the occurrence of local burn, which could be severe. Cold compresses should be applied to the injection site. Oral medications for pain relief may be administered.
5.2 Exposure to Sun or Direct Light
Following injection with VISUDYNE (verteporfin for injection), care should be taken to avoid exposure of skin or eyes to direct sunlight or bright indoor light for 5 days. In the event of extravasation during infusion, the extravasation area must be thoroughly protected from direct light until the swelling and discoloration have faded in order to prevent the occurrence of a local burn which could be severe. If emergency surgery is necessary within 48 hours after treatment, as much of the internal tissue as possible should be protected from intense light.
5.3 Decreased Vision After Treatment
Patients who experience severe decrease of vision of 4 lines or more within 1 week after treatment should not be retreated, at least until their vision completely recovers to pretreatment levels and the potential benefits and risks of subsequent treatment are carefully considered by the treating physician.
5.4 Anaphylactic Reactions
Cases of anaphylactic reactions have been reported in patients receiving VISUDYNE. Medical supervision is recommended during infusion. If an anaphylactic or other serious allergic reaction occurs during or following infusion, administration of VISUDYNE should be discontinued immediately and appropriate therapy should be initiated.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Local Adverse Reactions – Extravasation [see Warnings and Precautions (5.1)]
- Exposure to Sun or Direct Light [see Warnings and Precautions (5.2)]
- Decreased Vision after Treatment [see Warnings and Precautions (5.3)]
- Porphyria and Hypersensitivity [see Contraindications (4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Severe chest pain, vasovagal and hypersensitivity reactions have been reported. Vasovagal and hypersensitivity reactions on rare occasions can be severe. These reactions may include syncope, sweating, dizziness, rash, dyspnea, flushing and changes in blood pressure and heart rate. General symptoms can include headache, malaise, urticaria, and pruritus.
The most frequently reported adverse reactions to VISUDYNE (verteporfin for injection) are injection site reactions (including pain, edema, inflammation, extravasation, rashes, hemorrhage and discoloration) and visual disturbances (including blurred vision, flashes of light, decreased visual acuity and visual field defects, including scotoma). These events occurred in approximately 10%-30% of patients. The following events, listed by Body System, were reported more frequently with VISUDYNE therapy than with placebo therapy and occurred in 1%-10% of patients:
Ocular Treatment Site:
Blepharitis, cataracts, conjunctivitis/conjunctival injection, dry eyes, ocular itching, severe vision decrease with or without subretinal/retinal or vitreous hemorrhage
Body as a Whole:
Asthenia, fever, flu syndrome, infusion related pain primarily presenting as back pain, photosensitivity reactions
Cardiovascular:
Atrial fibrillation, hypertension, peripheral vascular disorder, varicose veins
Dermatologic:
Eczema
Digestive:
Constipation, gastrointestinal cancers, nausea
Hemic and Lymphatic:
Anemia, white blood cell count decreased, white blood cell count increased
Hepatic:
Elevated liver function tests
Metabolic/Nutritional:
Albuminuria, creatinine increased
Musculoskeletal:
Arthralgia, arthrosis, myasthenia
Nervous System:
Hypesthesia, sleep disorder, vertigo
Respiratory:
Cough, pharyngitis, pneumonia
Special Senses:
Cataracts, decreased hearing, diplopia, lacrimation disorder
Urogenital:
Prostatic disorder
Severe vision decrease, equivalent of >4 lines, within 7 days after treatment has been reported in 1%-5% of patients. Partial recovery of vision was observed in some patients. Photosensitivity reactions usually occurred in the form of skin sunburn following exposure to sunlight. The higher incidence of back pain in the VISUDYNE group occurred primarily during infusion.
The following adverse events have occurred either at low incidence (<1%) during clinical trials or have been reported during the use of VISUDYNE in clinical practice where these reactions were reported voluntarily from a population of unknown size and frequency of occurrence cannot be determined precisely. They have been chosen for inclusion based on factors such as seriousness, frequency of reporting, possible causal connection to VISUDYNE, or a combination of these factors:
Ocular Treatment Site:
Retinal detachment (nonrhegmatogenous), retinal or choroidal vessel nonperfusion, retinal pigment epithelial tear.
Non-ocular Events:
Chest pain and other musculoskeletal pain during infusion, anaphylactic
reaction during or following infusion, injection site necrosis.
-
7 DRUG INTERACTIONS
Drug interaction studies in humans have not been conducted with VISUDYNE.
Verteporfin is rapidly eliminated by the liver, mainly as unchanged drug. Metabolism is limited and occurs by liver and plasma esterases. Microsomal cytochrome P450 does not appear to play a role in verteporfin metabolism.
Based on the mechanism of action of verteporfin, many drugs used concomitantly could influence the effect of VISUDYNE therapy. Possible examples include the following:
Calcium channel blockers, polymyxin B or radiation therapy could enhance the rate of VISUDYNE uptake by the vascular endothelium. Other photosensitizing agents (e.g., tetracyclines, sulfonamides, phenothiazines, sulfonylurea hypoglycemic agents, thiazide diuretics and griseofulvin) could increase the potential for skin photosensitivity reactions. Compounds that quench active oxygen species or scavenge radicals, such as dimethyl sulfoxide, β-carotene, ethanol, formate and mannitol, would be expected to decrease VISUDYNE activity. Drugs that decrease clotting, vasoconstriction or platelet aggregation, e.g., thromboxane A2 inhibitors, could also decrease the efficacy of VISUDYNE therapy.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with the use of VISUDYNE in pregnant women to inform a drug-associated risk. Intravenous administration of verteporfin to pregnant rats during the period of organogenesis produced an increase in the incidence of anophthalmia/microphthalmia and wavy ribs at exposures approximately 40-fold the human exposure at the recommended clinical dose.
Verteporfin did not produce adverse fetal effect in rats or rabbits at exposures 6- to 20-fold the human exposure at the recommended clinical dose.
There are no adequate and well-controlled studies in pregnant women. VISUDYNE should be used during pregnancy only if the benefit justifies the potential risk to the fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Data
Animal Data
Rat fetuses of dams administered verteporfin for injection intravenously during organogenesis exhibited an increase in the incidence of anophthalmia/microphthalmia and wavy ribs at doses ≥10 mg/kg/day (approximately 40-fold the human exposure at the recommended dose of 6 mg/m2, based on AUC in female rats). No teratogenic effects were observed in rat fetuses at a dose of 2 mg/kg/day (approximately 6-fold the human exposure at the recommended dose of 6 mg/m2, based on AUC in female rats).
In pregnant rabbits, a decrease in maternal body weight gain and food consumption was observed in animals that received verteporfin for injection intravenously at doses up to 10 mg/kg/day during organogenesis. The no observed adverse effect level (NOAEL) for maternal toxicity was 3 mg/kg/day (approximately 6-fold the recommended human dose of 6 mg/m2, based on body surface area). No teratogenic effects were observed in rabbit fetuses at doses up to 10 mg/kg/day (approximately 20-fold the recommended human dose of 6 mg/m2, based on body surface area).
8.2 Lactation
Risk Summary
Verteporfin and its diacid metabolite have been found in human breast milk following an intravenous infusion at the recommended human dose of 6 mg/m2. Verteporfin was present in breast milk at levels up to 66% of the corresponding plasma levels and declined below the limit of quantification (2 ng/mL) within 24 hours. The diacid metabolite had lower peak concentrations but persisted up to at least 48 hours.
Because of the potential for serious adverse reactions in nursing infants from VISUDYNE, a decision should be made whether to discontinue nursing or postpone treatment, taking into account the importance of the drug to the mother.
-
10 OVERDOSAGE
Overdose of drug and/or light in the treated eye may result in non-perfusion of normal retinal vessels with the possibility of severe decrease in vision that could be permanent. An overdose of drug will also result in the prolongation of the period during which the patient remains photosensitive to bright light. In such cases, it is recommended to extend the photosensitivity precautions for a time proportional to the overdose.
-
11 DESCRIPTION
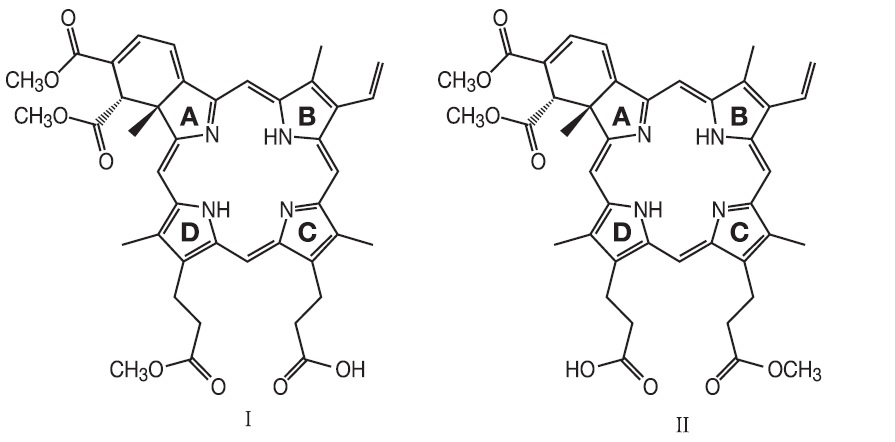
VISUDYNE® (verteporfin for injection) is a sterile, photoenhancer which is light activated drug when used in photodynamic therapy for intravenous administration. The finished drug product is a lyophilized dark green cake. Verteporfin is a 1:1 mixture of two regioisomers (I and II), represented by the following structures:
The chemical names for the verteporfin regioisomers are:
9-methyl (I) and 13-methyl (II) trans-(±)-18-ethenyl-4,4a,-dihydro-3,4-bis(methoxycarbonyl)-4a,8,14,19-tetramethyl-23H, 25H-benzo[b]porphine-9,13-dipropanoate
The molecular formula is C41H42N4O8 with a molecular weight of approximately 718.8. Each mL of reconstituted VISUDYNE contains:
ACTIVE:
verteporfin, 2 mg
INACTIVES:
ascorbyl palmitate (0.02 mg), butylated hydroxytoluene (0.002 mg), dimyristoyl phosphatidylcholine (9.4 mg), egg phosphatidylglycerol (6.5 mg), and lactose (92.0 mg).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
VISUDYNE (verteporfin for injection) therapy is a two-stage process requiring administration of both verteporfin for injection and nonthermal red light.
Verteporfin is transported in the plasma primarily by lipoproteins. Once verteporfin is activated by light in the presence of oxygen, highly reactive, short-lived singlet oxygen and reactive oxygen radicals are generated. Light activation of verteporfin results in local damage to neovascular endothelium, resulting in vessel occlusion. Damaged endothelium is known to release procoagulant and vasoactive factors through the lipoxygenase (leukotriene) and cyclooxygenase (eicosanoids such as thromboxane) pathways, resulting in platelet aggregation, fibrin clot formation and vasoconstriction. Verteporfin appears to somewhat preferentially accumulate in neovasculature, including choroidal neovasculature. However, animal models indicate that the drug is also present in the retina. Therefore, there may be collateral damage to retinal structures following photoactivation including the retinal pigmented epithelium and outer nuclear layer of the retina. The temporary occlusion of the CNV following VISUDYNE therapy has been confirmed in humans by fluorescein angiography.
12.3 Pharmacokinetics
Following intravenous infusion, verteporfin exhibits a bi-exponential elimination with a terminal elimination half-life of approximately 5-6 hours. The extent of exposure and the maximal plasma concentration are proportional to the dose between 6 and 20 mg/m2. At the intended dose, pharmacokinetic parameters are not significantly affected by gender.
Verteporfin is metabolized to a small extent to its diacid metabolite by liver and plasma esterases. NADPH-dependent liver enzyme systems (including the cytochrome P450 isozymes) do not appear to play a role in the metabolism of verteporfin. Elimination is by the fecal route, with less than 0.01% of the dose recovered in urine.
In a study of patients with mild hepatic insufficiency (defined as having two abnormal hepatic function tests at enrollment), AUC and Cmax were not significantly different from the control group; half-life, however, was significantly increased by approximately 20%.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No studies have been conducted to determine the carcinogenic potential of verteporfin.
Mutagenesis
Photodynamic therapy (PDT) as a class has been reported to result in DNA damage including DNA strand breaks, alkali-labile sites, DNA degradation, and DNA-protein cross-links which may result in chromosomal aberrations, sister chromatid exchanges (SCE), and mutations. In addition, other photodynamic therapeutic agents have been shown to increase the incidence of SCE in Chinese hamster ovary (CHO) cells irradiated with visible light and in Chinese hamster lung fibroblasts irradiated with near UV light, increase mutations and DNA-protein cross-linking in mouse L5178 cells, and increase DNA-strand breaks in malignant human cervical carcinoma cells, but not in normal cells. Verteporfin was not evaluated in these latter systems. It is not known how the potential for DNA damage with PDT agents translates into human risk.
Impairment of Fertility
No effect on male or female fertility has been observed in rats following intravenous administration of verteporfin for injection up to 10 mg/kg/day (approximately 60- and 40-fold human exposure at 6 mg/m2 based on AUC in male and female rats, respectively).
13.2 Animal Toxicology and/or Pharmacology
At a >10-fold higher dose given by bolus injection to sedated or anesthetized pigs, verteporfin caused severe hemodynamic effects, including death, probably as a result of complement activation. These effects were diminished or abolished by pretreatment with antihistamine and they were not seen in conscious nonsedated pigs.
-
14 CLINICAL STUDIES
14.1 Age-Related Macular Degeneration
Two adequate and well-controlled, double-masked, placebo-controlled, randomized studies were conducted in patients with classic-containing subfoveal CNV secondary to AMD. A total of 609 patients (VISUDYNE 402, placebo 207) were enrolled in these two studies. During these studies, retreatment was allowed every 3 months if fluorescein angiograms showed any recurrence or persistence of leakage. The placebo control (sham treatment) consisted of intravenous administration of Dextrose 5% in Water, followed by light application identical to that used for VISUDYNE therapy.
The difference between treatment groups statistically favored VISUDYNE at the 1-year and 2-year analyses for visual acuity endpoints.
The subgroup of patients with predominately classic CNV lesions was more likely to exhibit a treatment benefit (N=242; VISUDYNE 159, placebo 83).
Predominantly classic CNV lesions were defined as those in which the classic component comprised 50% or more of the area of the entire lesion. For the primary efficacy endpoint (percentage of patients who lost less than 3 lines of visual acuity), these patients showed a difference of approximately 28% between treatment groups at both Months 12 and 24 (67% for VISUDYNE patients compared to 40% for placebo patients, at Month 12; and 59% for VISUDYNE patients compared to 31% for placebo patients, at Month 24). Severe vision loss (≥6 lines of visual acuity from baseline) was experienced by 12% of VISUDYNE-treated patients compared to 34% of placebo-treated patients at Month 12, and by 15% of VISUDYNE-treated patients compared to 36% of placebo-treated patients at Month 24.
Patients with predominantly classic CNV lesions that did not contain occult CNV exhibited the greatest benefit (N=134; VISUDYNE 90, placebo 44). At 1 year, these patients demonstrated a 49% difference between treatment groups when assessed by the <3 lines-lost definition (77% vs. 27%).
Older patients (≥75 years), patients with dark irides, patients with occult lesions or patients with less than 50% classic CNV were less likely to benefit from VISUDYNE therapy.
The safety and efficacy of VISUDYNE beyond 2 years have not been demonstrated.
Based on the Treatment of Age Related Macular Degeneration with Photodynamic Therapy Study (TAP) extension study, the average number of treatments per year was 3.5 in the first year after diagnosis, 2.4 in the second, 1.3 in the third, 0.4 in the fourth and 0.1 in the fifth year.
14.2 Pathologic Myopia
One adequate and well-controlled, double-masked, placebo-controlled, randomized study was conducted in patients with subfoveal CNV secondary to pathologic myopia. A total of 120 patients (VISUDYNE 81, placebo 39) were enrolled in the study. The treatment dosing and retreatments were the same as in the AMD studies. The difference between treatment groups statistically favored VISUDYNE at the 1-year analysis but not at the 2-year analysis for visual acuity endpoints. For the primary efficacy endpoint (percentage of patients who lost less than 3 lines of visual acuity), patients at the 1-year timepoint showed a difference of approximately 19% between treatment groups (86% for VISUDYNE patients compared to 67% for placebo patients). However, by the 2-year timepoint, the effect was no longer statistically significant (79% for VISUDYNE patients compared to 72% for placebo patients).
Based on the Verteporfin in Photodynamic Therapy in Pathologic Myopia (VIP-PM) extension study in pathologic myopia, the average number of treatments per year was 3.5 in the first year after diagnosis, 1.8 in the second, 0.4 in the third, 0.2 in the fourth and 0.1 in the fifth.
14.3 Presumed Ocular Histoplasmosis
One open-label study was conducted in patients with subfoveal CNV secondary to presumed ocular histoplasmosis. A total of 26 patients were treated with VISUDYNE in the study. The treatment dosing and retreatments for VISUDYNE were the same as the AMD studies. VISUDYNE-treated patients compare favorably with historical control data demonstrating a reduction in the number of episodes of severe visual acuity loss (>6 lines of loss).
Based on the VISUDYNE Ocular Histoplasmosis extension study in presumed ocular histoplasmosis, the average number of treatments per year was 2.9 in the first year after diagnosis, 1.2 in the second, 0.2 in the third and 0.1 in the fourth.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
VISUDYNE® (verteporfin for injection) is supplied in a single-dose glass vial with a gray bromobutyl stopper and aluminum flip-off cap. It contains a lyophilized dark green cake with 15 mg verteporfin.
- NDC 24208-560-15
Store VISUDYNE at controlled room temperature between 20°C to 25°C (68°F to 77°F). Protect from light.
16.1 Spills and Disposal
Spills of VISUDYNE should be wiped up with a damp cloth. Skin and eye contact should be avoided due to the potential for photosensitivity reactions upon exposure to light. Use of rubber gloves and eye protection is recommended. All materials should be disposed of properly.
16.2 Accidental Exposure
Because of the potential to induce photosensitivity reactions, it is important to avoid contact with the eyes and skin during preparation and administration of VISUDYNE. Any exposed person must be protected from bright light [seeWarnings and Precautions (5.1)].
-
17 PATIENT COUNSELING INFORMATION
Advise patients who receive VISUDYNE that they will become temporarily photosensitive after the infusion. Patients should be advised to wear a wristband to remind them to avoid direct sunlight for 5 days. During that period, patients should be advised to avoid exposure of unprotected skin, eyes or other body organs to direct sunlight or bright indoor light. Sources of bright light include, but are not limited to, tanning salons, bright halogen lighting and high power lighting used in surgical operating rooms or dental offices. Prolonged exposure to light from light-emitting medical devices such as pulse oximeters should also be avoided for 5 days following VISUDYNE administration.
If treated patients must go outdoors in daylight during the first 5 days after treatment, they should be advised to protect all parts of their skin and their eyes by wearing protective clothing and dark sunglasses. UV sunscreens are not effective in protecting against photosensitivity reactions because photoactivation of the residual drug in the skin can be caused by visible light.
Patients should be advised to not stay in the dark and should be encouraged to expose their skin to ambient indoor light, as it will help inactivate the drug in the skin through a process called photobleaching.
Following VISUDYNE treatment, patients should be advised that they may develop visual disturbances such as abnormal vision, vision decrease, or visual field defects that may interfere with their ability to drive or use machines. Patients should be advised to not drive or use machines as long as these symptoms persist.
Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Manufactured by:
Alcami Carolinas Corporation
Charleston, SC 29405 USAVISUDYNE is a registered trademark of CHEPLAPHARM ARZNEIMITTEL GMBH used under license.
Other product/brand names are trademarks of the respective owners.
© 2023 Bausch & Lomb Incorporated or its affiliates
9589703
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VISUDYNE
verteporfin for injection injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 24208-560 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VERTEPORFIN (UNII: 0X9PA28K43) (VERTEPORFIN - UNII:0X9PA28K43) VERTEPORFIN 15 mg Inactive Ingredients Ingredient Name Strength ASCORBYL PALMITATE (UNII: QN83US2B0N) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 24208-560-15 1 in 1 CARTON; Type 0: Not a Combination Product 02/27/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021119 04/12/2000 Labeler - Bausch & Lomb Incorporated (196603781) Establishment Name Address ID/FEI Business Operations Alcami Carolinas Corporation 832394733 MANUFACTURE(24208-560) , PACK(24208-560)
Trademark Results [Visudyne]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VISUDYNE 75507102 2498651 Live/Registered |
CHEPLAPHARM ARZNEIMITTEL GMBH 1998-06-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.