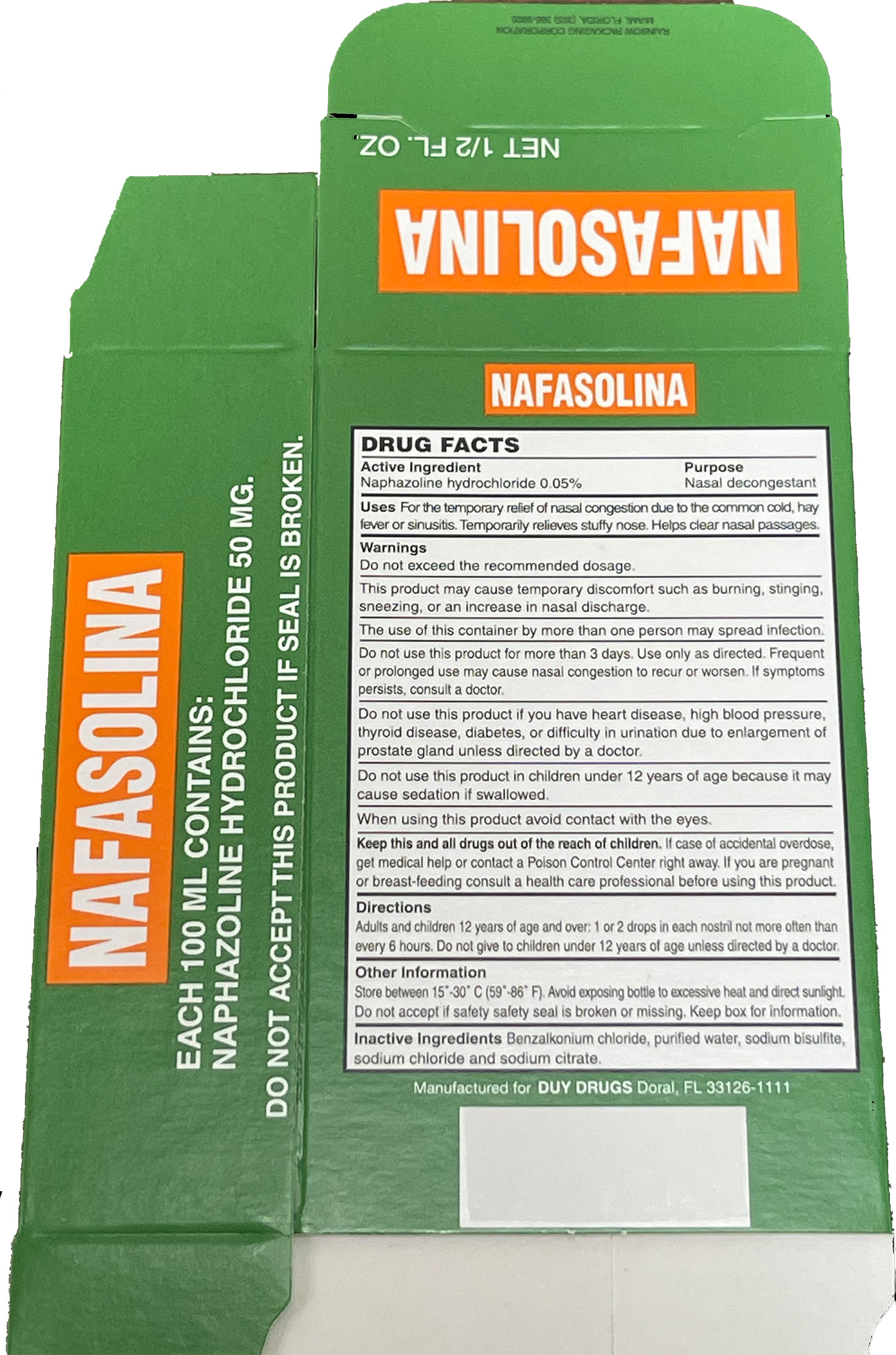
Naphazoline hydrochloride 0.05%
Naphazoline hydrochloride by
Drug Labeling and Warnings
Naphazoline hydrochloride by is a Otc medication manufactured, distributed, or labeled by Doral Pharmamedics Inc DBA AG Marin Pharmaceuticals, DEXTRUM LABORATORIES INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NAPHAZOLINE HYDROCHLORIDE- naphazoline hydrochloride solution/ drops
Doral Pharmamedics Inc DBA AG Marin Pharmaceuticals
----------
Naphazoline hydrochloride 0.05%
Keep out of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away.
Stop using this product after 3 days.
If symptoms persist, stop, and consult a doctor.
For the temporary relief of nasal congestion due to the common cold, hay fever, or sinusitis.
Temporarily relieves a stuffy nose.
Helps clear nasal passages.
Adults and children 12 years of age and over: 1 or 2 drops in each nostril not more often than every 6 hours. Do not give to children under 12 years of age unless directed by a doctor.
Do not exceed recommended dosage.
This product may cause temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge.
The use of this container by more than one person may spread infection.
Do not use this product for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen. If symptoms persist, consult a doctor.
Do not use this product if you have heart disease, high blood pressure, thyroid disease, diabetes, or difficulty in urination due to enlargement of the prostate gland unless directed by a doctor.
Do not use this product in children under 12 years of age because it may cause sedation if swallowed.
When using this product avoid contact with the eyes.
If you are pregnant or breast-feeding consult a health care professional before using this product.
Do not use this product if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2
weeks after stopping the MAOI drug. If you are uncertain whether your prescription drug contains an MAOI, consult a health professional before taking this product.
Do not use this product for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen. If symptoms persist, consult a doctor.
Do not use this product if you have heart disease, high blood pressure, thyroid disease, diabetes, or difficulty in urination due to enlargement of the prostate gland unless directed by a doctor.
Do not use this product in children under 12 years of age because it may cause sedation if swallowed.
Do not use this product in a child who has heart disease, high blood pressure, thyroid disease, or diabetes unless directed by a doctor.
This product is for nasal use only.
When using this product avoid contact with the eyes. In case of contact with eyes, rinse eyes thoroughly with water.
| NAPHAZOLINE HYDROCHLORIDE
naphazoline hydrochloride solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Doral Pharmamedics Inc DBA AG Marin Pharmaceuticals (076007996) |
| Registrant - Doral Pharmamedics Inc DBA AG Marin Pharmaceuticals (076007996) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DEXTRUM LABORATORIES INC. | 007392322 | manufacture(12539-145) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.