These highlights do not include all the information needed to use PITAVASTATIN TABLETS safely and effectively. See full prescribing information for PITAVASTATIN TABLETS.PITAVASTATIN Tablets, for oral useInitial U.S. Approval: 2009
Pitavastatin by
Drug Labeling and Warnings
Pitavastatin by is a Prescription medication manufactured, distributed, or labeled by QUINN PHARMACEUTICALS, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PITAVASTATIN- pitavastatin calcium tablet, film coated
QUINN PHARMACEUTICALS, LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PITAVASTATIN TABLETS safely and effectively. See full prescribing information for PITAVASTATIN TABLETS.
PITAVASTATIN Tablets, for oral use Initial U.S. Approval: 2009 INDICATIONS AND USAGEPitavastatin tablets is a HMG-CoA reductase inhibitor indicated for:
Limitations of Use (1.2):
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most frequent adverse reactions (rate ≥2.0% in at least one marketed dose) were myalgia, back pain, diarrhea, constipation and pain in extremity. (6) To report SUSPECTED ADVERSE REACTIONS, contact Quinn Pharmaceuticals, LLC at 1-800-244-0277 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2017 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Drug therapy should be one component of multiple-risk-factor intervention in individuals who require modifications of their lipid profile. Lipid-altering agents should be used in addition to a diet restricted in saturated fat and cholesterol only when the response to diet and other nonpharmacological measures has been inadequate.
1.1 Primary Hyperlipidemia and Mixed Dyslipidemia
Pitavastatin tablets is indicated as an adjunctive therapy to diet to reduce elevated total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), triglycerides (TG), and to increase HDL-C in adult patients with primary hyperlipidemia or mixed dyslipidemia.
1.2 Limitations of Use
Doses of pitavastatin tablets greater than 4 mg once daily were associated with an increased risk for severe myopathy in premarketing clinical studies. Do not exceed 4 mg once daily dosing of pitavastatin tablets.
The effect of pitavastatin tablets on cardiovascular morbidity and mortality has not been determined.
Pitavastatin tablets has not been studied in Fredrickson Type I, III, and V dyslipidemias.
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
The dose range for pitavastatin tablets is 1 mg to 4 mg orally once daily at any time of the day with or without food. The recommended starting dose is 2 mg and the maximum dose is 4 mg. The starting dose and maintenance doses of pitavastatin tablets should be individualized according to patient characteristics, such as goal of therapy and response.
After initiation or upon titration of pitavastatin tablets, lipid levels should be analyzed after 4 weeks and the dosage adjusted accordingly.
2.2 Dosage in Patients with Renal Impairment
Patients with moderate and severe renal impairment (glomerular filtration rate 30 to 59 mL/min/1.73 m2 and 15 to 29 mL/min/1.73 m2 not receiving hemodialysis, respectively) as well as end-stage renal disease receiving hemodialysis should receive a starting dose of pitavastatin tablets 1 mg once daily and a maximum dose of pitavastatin tablets 2 mg once daily.
2.3 Use with Erythromycin
In patients taking erythromycin, a dose of pitavastatin tablets 1 mg once daily should not be exceeded [see Drug Interactions (7.2)].
2.4 Use with Rifampin
In patients taking rifampin, a dose of pitavastatin tablets 2 mg once daily should not be exceeded [see Drug Interactions (7.3)].
3 DOSAGE FORMS AND STRENGTHS
1 mg: Round white to off-white film-coated tablet debossed with "P1" on one side
2 mg: Round white to off-white film-coated tablet debossed with "P2" on one side
4 mg: Round white to off-white film-coated tablet debossed with "P4" on one side
4 CONTRAINDICATIONS
The use of pitavastatin tablets is contraindicated in the following conditions:
- Patients with a known hypersensitivity to any component of this product. Hypersensitivity reactions including rash, pruritus, and urticaria have been reported with pitavastatin tablets [see Adverse Reactions (6.1)].
- Patients with active liver disease which may include unexplained persistent elevations of hepatic transaminase levels [see Warnings and Precautions (5.2), Use in Specific Populations (8.7)].
- Co-administration with cyclosporine [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
- Pregnancy. [see Use in Specific Populations (8.1, 8.3)].
- Lactation. It is not known if pitavastatin is present in human milk; however, another drug in this class passes into breast milk. Since HMG-CoA reductase inhibitors have the potential for serious adverse reactions in breastfed infants, women who require pitavastatin treatment should not breastfeed their infants [see Use in Specific Populations (8.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Skeletal Muscle Effects
Cases of myopathy and rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with HMG-CoA reductase inhibitors, including Pitavastatin Tablets. These risks can occur at any dose level, but increase in a dose-dependent manner.
Pitavastatin tablets should be prescribed with caution in patients with predisposing factors for myopathy. These factors include advanced age (≥65 years), renal impairment, and inadequately treated hypothyroidism. The risk of myopathy may also be increased with concurrent administration of fibrates or lipid-modifying doses of niacin. Pitavastatin tablets should be administered with caution in patients with impaired renal function, in elderly patients, or when used concomitantly with fibrates or lipid-modifying doses of niacin [see Drug Interactions (7.6), Use in Specific Populations (8.5, 8.6) and Clinical Pharmacology (12.3)].
Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA reductase inhibitors coadministered with colchicine, and caution should be exercised when prescribing pitavastatin tablets with colchicine [see Drug Interactions (7.7].
There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; muscle biopsy showing necrotizing myopathy without significant inflammation; improvement with immunosuppressive agents.
Pitavastatin tablets therapy should be discontinued if markedly elevated creatine kinase (CK) levels occur or myopathy is diagnosed or suspected. Pitavastatin tablets therapy should also be temporarily withheld in any patient with an acute, serious condition suggestive of myopathy or predisposing to the development of renal failure secondary to rhabdomyolysis (e.g., sepsis, hypotension, dehydration, major surgery, trauma, severe metabolic, endocrine, and electrolyte disorders, or uncontrolled seizures). All patients should be advised to promptly report unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing pitavastatin tablets.
5.2 Liver Enzyme Abnormalities
Increases in serum transaminases (aspartate aminotransferase [AST]/serum glutamic-oxaloacetic transaminase, or alanine aminotransferase [ALT]/serum glutamic-pyruvic transaminase) have been reported with HMG-CoA reductase inhibitors, including pitavastatin tablets. In most cases, the elevations were transient and resolved or improved on continued therapy or after a brief interruption in therapy.
In placebo-controlled Phase 2 studies, ALT >3 times the upper limit of normal was not observed in the placebo, pitavastatin tablets 1 mg, or pitavastatin tablets 2 mg groups. One out of 202 patients (0.5%) administered pitavastatin tablets 4 mg had ALT >3 times the upper limit of normal.
It is recommended that liver enzyme tests be performed before the initiation of pitavastatin tablets and if signs or symptoms of liver injury occur. All patients treated with pitavastatin tablets should be advised to report promptly any symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice.
There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including pitavastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with pitavastatin tablets, promptly interrupt therapy. If an alternate etiology is not found do not restart pitavastatin tablets.
As with other HMG-CoA reductase inhibitors, pitavastatin tablets should be used with caution in patients who consume substantial quantities of alcohol. Active liver disease, which may include unexplained persistent transaminase elevations, is a contraindication to the use of pitavastatin tablets [see Contraindications (4)].
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Rhabdomyolysis with myoglobinuria and acute renal failure and myopathy (including myositis) [see Warnings and Precautions (5.1)].
- Liver Enzyme Abnormalities [see Warning and Precautions (5.2)].
Of 4,798 patients enrolled in 10 controlled clinical studies and 4 subsequent open-label extension studies, 3,291 patients were administered pitavastatin 1 mg to 4 mg daily. The mean continuous exposure of pitavastatin (1 mg to 4 mg) was 36.7 weeks (median 51.1 weeks). The mean age of the patients was 60.9 years (range; 18 years – 89 years) and the gender distribution was 48% males and 52% females. Approximately 93% of the patients were Caucasian, 7% were Asian/Indian, 0.2% were African American and 0.3% were Hispanic and other.
6.1 Clinical Studies Experience
Because clinical studies on pitavastatin tablets are conducted in varying study populations and study designs, the frequency of adverse reactions observed in the clinical studies of pitavastatin tablets cannot be directly compared with that in the clinical studies of other HMG-CoA reductase inhibitors and may not reflect the frequency of adverse reactions observed in clinical practice.
Adverse reactions reported in ≥ 2% of patients in controlled clinical studies and at a rate greater than or equal to placebo are shown in Table 1. These studies had treatment duration of up to 12 weeks.
|
|
||||
| Adverse Reactions* | Placebo N= 208 |
Pitavastatin Tablets 1 mg |
Pitavastatin Tablets |
Pitavastatin Tablets |
| Back Pain | 2.9% | 3.9% | 1.8% | 1.4% |
| Constipation | 1.9% | 3.6% | 1.5% | 2.2% |
| Diarrhea | 1.9% | 2.6% | 1.5% | 1.9% |
| Myalgia | 1.4% | 1.9% | 2.8% | 3.1% |
| Pain in extremity | 1.9% | 2.3% | 0.6% | 0.9% |
Other adverse reactions reported from clinical studies were arthralgia, headache, influenza, and nasopharyngitis.
The following laboratory abnormalities have also been reported: elevated creatine phosphokinase, transaminases, alkaline phosphatase, bilirubin, and glucose.
In controlled clinical studies and their open-label extensions, 3.9% (1 mg), 3.3% (2 mg), and 3.7% (4 mg) of pitavastatin-treated patients were discontinued due to adverse reactions. The most common adverse reactions that led to treatment discontinuation were: elevated creatine phosphokinase (0.6% on 4 mg) and myalgia (0.5% on 4 mg).
Hypersensitivity reactions including rash, pruritus, and urticaria have been reported with pitavastatin tablets.
In a double-blind, randomized, controlled, 52-week trial, 252 HIV-infected patients with dyslipidemia were treated with either pitavastatin tablets 4mg once daily (n=126) or another statin (n=126). All patients were taking antiretroviral therapy (excluding darunavir) and had HIV-1 RNA less than 200 copies/mL and CD4 count greater than 200 cell/μL for at least 3 months prior to randomization. The safety profile of pitavastatin tablets was generally consistent with that observed in the clinical trials described above. One patient (0.8%) treated with pitavastatin tablets had a peak creatine phosphokinase value exceeding 10 times the upper limit of normal (10x ULN), which resolved spontaneously. Four patients (3%) treated with pitavastatin tablets had at least one ALT value exceeding 3x but less than 5x ULN, none of which led to drug discontinuation. Virologic failure was reported for four patients (3%) treated with pitavastatin tablets, defined as a confirmed measurement of HIV-1 RNA exceeding 200 copies/mL that was also more than a 2-fold increase from baseline.
6.2 Postmarketing Experience:
The following adverse reactions have been identified during post approval use of pitavastatin tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions associated with pitavastatin tablets therapy reported since market introduction, regardless of causality assessment, include the following: abdominal discomfort, abdominal pain, dyspepsia, nausea, asthenia, fatigue, malaise, hepatitis, jaundice, fatal and non-fatal hepatic failure, dizziness, hypoesthesia, insomnia, depression, interstitial lung disease, erectile dysfunction and muscle spasms.
There have been rare postmarketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).
There have been rare reports of immune-mediated necrotizing myopathy associated with statin use [see Warnings and Precautions (5.1)].
7 DRUG INTERACTIONS
7.1 Cyclosporine
Cyclosporine significantly increased pitavastatin exposure. Co-administration of cyclosporine with pitavastatin tablets is contraindicated [see Contraindications (4), and Clinical Pharmacology (12.3)].
7.2 Erythromycin
Erythromycin significantly increased pitavastatin exposure. In patients taking erythromycin, a dose of pitavastatin tablets 1 mg once daily should not be exceeded [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
7.3 Rifampin
Rifampin significantly increased pitavastatin exposure. In patients taking rifampin, a dose of pitavastatin tablets 2 mg once daily should not be exceeded [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
7.4 Gemfibrozil
Due to an increased risk of myopathy/rhabdomyolysis when HMG-CoA reductase inhibitors are coadministered with gemfibrozil, concomitant administration of pitavastatin tablets with gemfibrozil should be avoided.
7.5 Other Fibrates
Because it is known that the risk of myopathy during treatment with HMG-CoA reductase inhibitors is increased with concurrent administration of other fibrates, pitavastatin tablets should be administered with caution when used concomitantly with other fibrates [see Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)].
7.6 Niacin
The risk of skeletal muscle effects may be enhanced when pitavastatin tablets is used in combination with niacin; a reduction in pitavastatin tablets dosage should be considered in this setting [see Warnings and Precautions (5.1)].
7.7 Colchicine
Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA reductase inhibitors coadministered with colchicine, and caution should be exercised when prescribing pitavastatin tablets with colchicine.
7.8 Warfarin
Pitavastatin tablets had no significant pharmacokinetic interaction with R- and S- warfarin. Pitavastatin tablets had no significant effect on prothrombin time (PT) and international normalized ratio (INR) when administered to patients receiving chronic warfarin treatment [see Clinical Pharmacology (12.3)]. However, patients receiving warfarin should have their PT and INR monitored when pitavastatin is added to their therapy.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Pitavastatin tablets is contraindicated for use in pregnant women since safety in pregnant women has not been established and there is no apparent benefit to therapy with pitavastatin tablets during pregnancy. Because HMGCoA reductase inhibitors decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, pitavastatin tablets may cause fetal harm when administered to pregnant women. pitavastatin tablets should be discontinued as soon as pregnancy is recognized [see Contraindications (4). Limited published data on the use of pitavastatin tablets are insufficient to determine a drug-associated risk of major congenital malformations or miscarriage. In animal reproduction studies, no embryo-fetal toxicity or congenital malformations were observed when pregnant rats and rabbits were orally administered pitavastatin during organogenesis at exposures which were 22 and 4 times, respectively, the maximum recommended human dose (MRHD) [see Data]. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Human Data
Limited published data on pitavastatin tabletsS have not reported a drug-associated risk of major congenital malformations or miscarriage. Rare reports of congenital anomalies have been received following intrauterine exposure to HMG-CoA reductase inhibitors. In a review of about 100 prospectively followed pregnancies in women exposed to other HMG-CoA reductase inhibitors, the incidences of congenital anomalies, spontaneous abortions, and fetal deaths/stillbirths did not exceed the rate expected in the general population. The number of cases is adequate to exclude a greater than or equal to a 3- to 4-fold increase in congenital anomalies over background incidence. In 89% of the prospectively followed pregnancies, drug treatment was initiated prior to pregnancy and was discontinued at some point in the first trimester when pregnancy was identified.
Animal Data
Reproductive toxicity studies have shown that pitavastatin crosses the placenta in rats and is found in fetal tissues at ≤36% of maternal plasma concentrations following a single dose of 1 mg/kg/day during gestation.
Embryo-fetal developmental studies were conducted in pregnant rats treated with 3, 10, 30 mg/kg/day pitavastatin by oral gavage during organogenesis. No adverse effects were observed at 3 mg/kg/day, systemic exposures 22 times human systemic exposure at 4 mg/day based on AUC.
Embryo-fetal developmental studies were conducted in pregnant rabbits treated with 0.1, 0.3, 1 mg/kg/day pitavastatin by oral gavage during the period of fetal organogenesis. Maternal toxicity consisting of reduced body weight and abortion was observed at all doses tested (4 times human systemic exposure at 4 mg/day based on AUC).
In perinatal/postnatal studies in pregnant rats given oral gavage doses of pitavastatin at 0.1, 0.3, 1, 3, 10, 30 mg/kg/day from organogenesis through weaning, maternal toxicity consisting of mortality at ≥0.3 mg/kg/day and impaired lactation at all doses contributed to the decreased survival of neonates in all dose groups (0.1 mg/kg/day represents approximately 1 time human systemic exposure at 4 mg/day dose based on AUC).
8.2 Lactation
Risk Summary
Pitavastatin tablets is contraindicated during breastfeeding [see Contraindications (4)] . There is no available information on the effects of the drug on the breastfed infant or the effects of the drug on milk production. However, it has been shown that another drug in this class passes into human milk. Because of the potential for serious adverse reactions in a breastfed infant, advise patients that breastfeeding is not recommended during treatment with pitavastatin tablets.
8.3 Females and Males of Reproductive Potential
Contraception
Females
Pitavastatin tablets may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with pitavastatin tablets.
8.4 Pediatric Use
Safety and effectiveness of pitavastatin tablets in pediatric patients have not been established.
8.5 Geriatric Use
Of the 2,800 patients randomized to pitavastatin tablets 1 mg to 4 mg in controlled clinical studies, 1,209 (43%) were 65 years and older. No significant differences in efficacy or safety were observed between elderly patients and younger patients. However, greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Patients with moderate and severe renal impairment (glomerular filtration rate 30 to 59 mL/min/1.73 m2 and 15 to 29 mL/min/1.73 m2 not receiving hemodialysis, respectively) as well as end-stage renal disease receiving hemodialysis should receive a starting dose of pitavastatin tablets 1 mg once daily and a maximum dose of pitavastatin tablets 2 mg once daily [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
There is no known specific treatment in the event of overdose of pitavastatin. In the event of overdose, the patient should be treated symptomatically and supportive measures instituted as required. Hemodialysis is unlikely to be of benefit due to high protein binding ratio of pitavastatin.
11 DESCRIPTION
Pitavastatin tabletsis an inhibitor of HMG-CoA reductase. It is a synthetic lipid-lowering agent for oral administration.
The chemical name for pitavastatin is (+)monocalcium bis{(3R, 5S, 6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-3,5-dihydroxy-6-heptenoate}. The structural formula is:
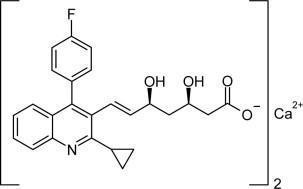
The empirical formula for pitavastatin is C50H46CaF2N2O8 and the molecular weight is 880.98. Pitavastatin is odorless and occurs as white to pale-yellow powder. It is freely soluble in pyridine, chloroform, dilute hydrochloric acid, and tetrahydrofuran, soluble in ethylene glycol, sparingly soluble in octanol, slightly soluble in methanol, very slightly soluble in water or ethanol, and practically insoluble in acetonitrile or diethyl ether. Pitavastatin is hygroscopic and slightly unstable in light.
Each film-coated tablet ofpitavastatin tablets contains 1.045 mg, 2.09 mg, or 4.18 mg of pitavastatin calcium, which is equivalent to 1 mg, 2 mg, or 4 mg, respectively of free base and the following inactive ingredients: lactose monohydrate, magnesium carbonate, low-substituted hydroxypropyl cellulose, hypromellose, magnesium stearate, purified water, and film coating containing the following inactive ingredients: hypromellose, purified water, polyethylene glycol, talc and titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pitavastatin competitively inhibits HMG-CoA reductase, which is a rate-determining enzyme involved with biosynthesis of cholesterol, in a manner of competition with the substrate so that it inhibits cholesterol synthesis in the liver. As a result, the expression of LDL-receptors followed by the uptake of LDL from blood to liver is accelerated and then the plasma TC decreases. Further, the sustained inhibition of cholesterol synthesis in the liver decreases levels of very low density lipoproteins.
12.2 Pharmacodynamics
In a randomized, double-blind, placebo-controlled, 4-way parallel, active-comparator study with moxifloxacin in 174 healthy participants, pitavastatin tablets was not associated with clinically meaningful prolongation of the QTc interval or heart rate at daily doses up to 16 mg (4 times the recommended maximum daily dose).
12.3 Pharmacokinetics
Absorption: Pitavastatin peak plasma concentrations are achieved about 1 hour after oral administration. Both Cmax and AUC0-inf increased in an approximately dose-proportional manner for single pitavastatin tablets doses from 1 to 24 mg once daily. The absolute bioavailability of pitavastatin oral solution is 51%. Administration of pitavastatin tablets with a high fat meal (50% fat content) decreases pitavastatin Cmax by 43% but does not significantly reduce pitavastatin AUC. The Cmax and AUC of pitavastatin did not differ following evening or morning drug administration. In healthy volunteers receiving 4 mg pitavastatin, the percent change from baseline for LDL-C following evening dosing was slightly greater than that following morning dosing. Pitavastatin was absorbed in the small intestine but very little in the colon.
Distribution: Pitavastatin is more than 99% protein bound in human plasma, mainly to albumin and alpha 1-acid glycoprotein, and the mean volume of distribution is approximately 148 L. Association of pitavastatin and/or its metabolites with the blood cells is minimal.
Metabolism: Pitavastatin is marginally metabolized by CYP2C9 and to a lesser extent by CYP2C8. The major metabolite in human plasma is the lactone which is formed via an ester-type pitavastatin glucuronide conjugate by uridine 5'-diphosphate (UDP) glucuronosyltransferase (UGT1A3 and UGT2B7).
Excretion: A mean of 15% of radioactivity of orally administered, single 32 mg 14C-labeled pitavastatin dose was excreted in urine, whereas a mean of 79% of the dose was excreted in feces within 7 days. The mean plasma elimination half-life is approximately 12 hours.
Race: In pharmacokinetic studies pitavastatin Cmax and AUC were 21 and 5% lower, respectively in Black or African American healthy volunteers compared with those of Caucasian healthy volunteers. In pharmacokinetic comparison between Caucasian volunteers and Japanese volunteers, there were no significant differences in Cmax and AUC.
Gender: In a pharmacokinetic study which compared healthy male and female volunteers, pitavastatin Cmax and AUC were 60 and 54% higher, respectively in females. This had no effect on the efficacy or safety of pitavastatin tablets in women in clinical studies.
Geriatric: In a pharmacokinetic study which compared healthy young and elderly (≥65 years) volunteers, pitavastatin Cmax and AUC were 10 and 30% higher, respectively, in the elderly. This had no effect on the efficacy or safety of pitavastatin tablets in elderly subjects in clinical studies.
Renal Impairment: In patients with moderate renal impairment (glomerular filtration rate of 30 to 59 mL/min/1.73 m2) and end stage renal disease receiving hemodialysis, pitavastatin AUC0-inf is 102 and 86% higher than those of healthy volunteers, respectively, while pitavastatin Cmax is 60 and 40% higher than those of healthy volunteers, respectively. Patients received hemodialysis immediately before pitavastatin dosing and did not undergo hemodialysis during the pharmacokinetic study. Hemodialysis patients have 33 and 36% increases in the mean unbound fraction of pitavastatin as compared to healthy volunteers and patients with moderate renal impairment, respectively.
In another pharmacokinetic study, patients with severe renal impairment (glomerular filtration rate 15 to 29 mL/min/1.73 m2) not receiving hemodialysis were administered a single dose of pitavastatin tablets 4 mg. The AUC0-inf and the Cmax were 36 and 18% higher, respectively, compared with those of healthy volunteers. For both patients with severe renal impairment and healthy volunteers, the mean percentage of protein-unbound pitavastatin was approximately 0.6%.
The effect of mild renal impairment on pitavastatin exposure has not been studied.
Hepatic Impairment: The disposition of pitavastatin was compared in healthy volunteers and patients with various degrees of hepatic impairment. The ratio of pitavastatin Cmax between patients with moderate hepatic impairment (Child-Pugh B disease) and healthy volunteers was 2.7. The ratio of pitavastatin AUCinf between patients with moderate hepatic impairment and healthy volunteers was 3.8. The ratio of pitavastatin Cmax between patients with mild hepatic impairment (Child-Pugh A disease) and healthy volunteers was 1.3. The ratio of pitavastatin AUCinf between patients with mild hepatic impairment and healthy volunteers was 1.6. Mean pitavastatin t½ for moderate hepatic impairment, mild hepatic impairment, and healthy were 15, 10, and 8 hours, respectively.
Drug-Drug Interactions: The principal route of pitavastatin metabolism is glucuronidation via liver UGTs with subsequent formation of pitavastatin lactone. There is only minimal metabolism by the cytochrome P450 system.
Warfarin: The steady-state pharmacodynamics (international normalized ratio [INR] and prothrombin time [PT]) and pharmacokinetics of warfarin in healthy volunteers were unaffected by the co-administration of pitavastatin tablets 4 mg daily. However, patients receiving warfarin should have their PT time or INR monitored when pitavastatin is added to their therapy.
|
|
|||
| Co-administered drug | Dose regimen | Change in AUC* | Change in Cmax* |
| Cyclosporine | Pitavastatin 2 mg QD for 6 days + cyclosporine 2 mg/kg on Day 6 | ↑ 4.6 fold† | ↑ 6.6 fold† |
| Erythromycin | Pitavastatin 4 mg single dose on Day 4 + erythromycin 500 mg 4 times daily for 6 days | ↑ 2.8 fold † | ↑ 3.6 fold † |
| Rifampin | Pitavastatin 4 mg QD + rifampin 600 mg QD for 5 days | ↑ 29% | ↑ 2.0 fold |
| Atazanavir | Pitavastatin 4 mg QD + atazanavir 300 mg daily for 5 days | ↑ 31% | ↑ 60% |
| Darunavir/Ritonavir | Pitavastatin 4mg QD on Days 1-5 and 12-16 + darunavir/ritonavir 800mg/100 mg QD on Days 6-16 | ↓ 26% | ↓ 4% |
| Lopinavir/Ritonavir | Pitavastatin 4 mg QD on Days 1-5 and 20-24 + lopinavir/ritonavir 400 mg/100 mg BID on Days 9 – 24 | ↓ 20% | ↓4 % |
| Gemfibrozil | Pitavastatin 4 mg QD + gemfibrozil 600 mg BID for 7 days | ↑ 45% | ↑ 31% |
| Fenofibrate | Pitavastatin 4 mg QD + fenofibrate 160 mg QD for 7 days | ↑18% | ↑ 11% |
| Ezetimibe | Pitavastatin 2 mg QD + ezetimibe 10 mg for 7 days | ↓ 2% | ↓0.2% |
| Enalapril | Pitavastatin 4 mg QD + enalapril 20 mg daily for 5 days | ↑ 6% | ↓ 7% |
| Digoxin | Pitavastatin 4 mg QD + digoxin 0.25 mg for 7 days | ↑ 4% | ↓ 9% |
| Diltiazem LA | Pitavastatin 4 mg QD on Days 1-5 and 11-15 and diltiazem LA 240 mg on Days 6-15 | ↑10% | ↑15% |
| Grapefruit Juice | Pitavastatin 2 mg single dose on Day 3 + grapefruit juice for 4 days | ↑ 15% | ↓ 12% |
| Itraconazole | Pitavastatin 4 mg single dose on Day 4 + itraconazole 200 mg daily for 5 days | ↓ 23% | ↓ 22% |
|
|
||||
| Co-administered drug | Dose regimen | Change in AUC** | Change in Cmax* | |
| Atazanavir | Pitavastatin 4 mg QD + atazanavir 300 mg daily for 5 days | ↑ 6% | ↑ 13% | |
| Darunavir | Pitavastatin 4mg QD on Days 1-5 and 12-16 + darunavir/ritonavir 800mg/100 mg QD on Days 6-16 | ↑ 3% | ↑ 6% | |
| Lopinavir | Pitavastatin 4 mg QD on Days 1-5 and 20-24 + lopinavir/ritonavir 400 mg/100 mg BID on Days 9 – 24 | ↓ 9% | ↓ 7% | |
| Ritonavir | Pitavastatin 4 mg QD on Days 1-5 and 20-24 + lopinavir/ritonavir 400 mg/100 mg BID on Days 9 – 24 | ↓ 11% | ↓ 11% | |
| Ritonavir | Pitavastatin 4mg QD on Days 1-5 and 12-16 + darunavir/ritonavir 800mg/100 mg QD on Days 6-16 | ↑ 8% | ↑ 2% | |
| Enalapril | Pitavastatin 4 mg QD + enalapril 20 mg daily for 5 days | Enalapril | ↑ 12% | ↑ 12% |
| Enalaprilat | ↓ 1% | ↓ 1% | ||
| Warfarin | Individualized maintenance dose of warfarin (2 - 7 mg) for 8 days + pitavastatin 4 mg QD for 9 days | R-warfarin | ↑ 7% | ↑ 3% |
| S-warfarin | ↑ 6% | ↑ 3% | ||
| Ezetimibe | Pitavastatin 2 mg QD + ezetimibe 10 mg for 7 days | ↑ 9% | ↑ 2% | |
| Digoxin | Pitavastatin 4 mg QD + digoxin 0.25 mg for 7 days | ↓ 3% | ↓ 4% | |
| Diltiazem LA | Pitavastatin 4 mg QD on Days 1-5 and 11-15 and diltiazem LA 240 mg on Days 6-15 | ↓ 2% | ↓ 7% | |
| Rifampin | Pitavastatin 4 mg QD + rifampin 600 mg QD for 5 days | ↓ 15% | ↓ 18% | |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 92-week carcinogenicity study in mice given pitavastatin, at the maximum tolerated dose of 75 mg/kg/day with systemic maximum exposures (AUC) 26 times the clinical maximum exposure at 4 mg/day, there was an absence of drug-related tumors.
In a 92-week carcinogenicity study in rats given pitavastatin at 1, 5, 25 mg/kg/day by oral gavage there was a significant increase in the incidence of thyroid follicular cell tumors at 25 mg/kg/day, which represents 295 times human systemic exposures based on AUC at the 4 mg/day maximum human dose.
In a 26-week transgenic mouse (Tg rasH2) carcinogenicity study where animals were given pitavastatin at 30, 75, and 150 mg/kg/day by oral gavage, no clinically significant tumors were observed.
Pitavastatin was not mutagenic in the Ames test with Salmonella typhimurium and Escherichia coli with and without metabolic activation, the micronucleus test following a single administration in mice and multiple administrations in rats, the unscheduled DNA synthesis test in rats, and a Comet assay in mice. In the chromosomal aberration test, clastogenicity was observed at the highest doses tested which also elicited high levels of cytotoxicity.
Pitavastatin had no adverse effects on male and female rat fertility at oral doses of 10 and 30 mg/kg/day, respectively, at systemic exposures 56- and 354-times clinical exposure at 4 mg/day based on AUC.
Pitavastatin treatment in rabbits resulted in mortality in males and females given 1 mg/kg/day (30-times clinical systemic exposure at 4 mg/day based on AUC) and higher during a fertility study. Although the cause of death was not determined, rabbits had gross signs of renal toxicity (kidneys whitened) indicative of possible ischemia. Lower doses (15-times human systemic exposure) did not show significant toxicity in adult males and females. However, decreased implantations, increased resorptions, and decreased viability of fetuses were observed.
13.2 Animal Toxicology and/or Pharmacology
Central Nervous System Toxicity
CNS vascular lesions, characterized by perivascular hemorrhages, edema, and mononuclear cell infiltration of perivascular spaces, have been observed in dogs treated with several other members of this drug class. A chemically similar drug in this class produced dose-dependent optic nerve degeneration (Wallerian degeneration of retinogeniculate fibers) in dogs, at a dose that produced plasma drug levels about 30 times higher than the mean drug level in humans taking the highest recommended dose. Wallerian degeneration has not been observed with pitavastatin. Cataracts and lens opacities were seen in dogs treated for 52 weeks at a dose level of 1 mg/kg/day (9 times clinical exposure at the maximum human dose of 4 mg/day based on AUC comparisons.
14 CLINICAL STUDIES
14.1 Primary Hyperlipidemia or Mixed Dyslipidemia
Dose-ranging study: A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study was performed to evaluate the efficacy of pitavastatin tablets compared with placebo in 251 patients with primary hyperlipidemia (Table 4). Pitavastatin tablets given as a single daily dose for 12 weeks significantly reduced plasma LDL-C, TC, TG, and Apo-B compared to placebo and was associated with variable increases in HDL-C across the dose range.
|
|
||||||
| Treatment | N | LDL-C | Apo-B | TC | TG | HDL-C |
| Placebo | 53 | -3 | -2 | -2 | 1 | 0 |
| Pitavastatin Tablets 1mg | 52 | -32 | -25 | -23 | -15 | 8 |
| PitavastatinTablets 2mg | 49 | -36 | -30 | -26 | -19 | 7 |
| PitavastatinTablets 4mg | 51* | -43 | -35 | -31 | -18 | 5 |
Active-controlled study with atorvastatin (NK-104-301): Pitavastatin tablets was compared with the HMG-CoA reductase inhibitor atorvastatin in a randomized, multicenter, double-blind, double-dummy, active-controlled, non-inferiority Phase 3 study of 817 patients with primary hyperlipidemia or mixed dyslipidemia. Patients entered a 6- to 8-week wash-out/dietary lead-in period and then were randomized to a 12-week treatment with either pitavastatin tablets or atorvastatin (Table 5). Non-inferiority of pitavastatin to a given dose of atorvastatin was considered to be demonstrated if the lower bound of the 95% CI for the mean treatment difference was greater than -6% for the mean percent change in LDL-C.
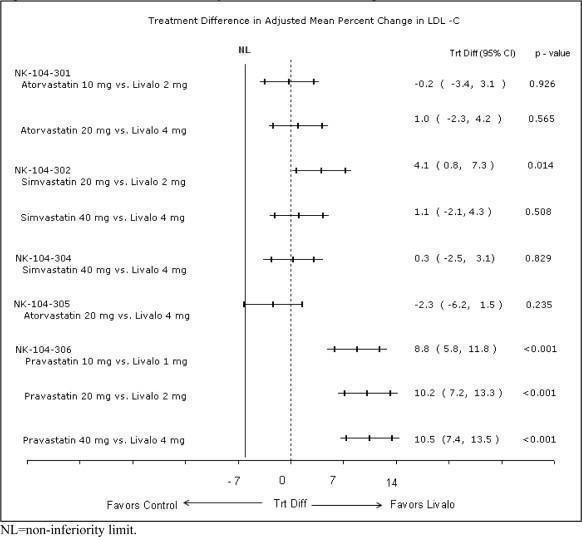
Lipid results are shown in Table 5. For the percent change from baseline to endpoint in LDL-C, pitavastatin tablets was non-inferior to atorvastatin for the two pairwise comparisons: Pitavastatin tablets 2 mg vs. atorvastatin 10 mg and pitavastatin tablets 4 mg vs. atorvastatin 20 mg. Mean treatment differences (95% CI) were 0% (-3%, 3%) and 1% (-2%, 4%), respectively.
| Treatment | N | LDL-C | Apo-B | TC | TG | HDL-C | non-HDL-C |
| Pitavastatin Tablets 2 mg daily | 315 | -38 | -30 | -28 | -14 | 4 | -35 |
| Pitavastatin Tablets 4 mg daily | 298 | -45 | -35 | -32 | -19 | 5 | -41 |
| Atorvastatin 10 mg daily | 102 | -38 | -29 | -28 | -18 | 3 | -35 |
| Atorvastatin 20 mg daily | 102 | -44 | -36 | -33 | -22 | 2 | -41 |
| Atorvastatin 40 mg daily Atorvastatin 80 mg daily |
-----------------------------------------Not Studied---------------------------------------------------------- ----------------------------------------Not Studied--------------------------------------------------------- |
||||||
Active-controlled study with simvastatin (NK-104-302): Pitavastatin tablets was compared with the HMG-CoA reductase inhibitor simvastatin in a randomized, multicenter, double-blind, double-dummy, active-controlled, non-inferiority Phase 3 study of 843 patients with primary hyperlipidemia or mixed dyslipidemia. Patients entered a 6- to 8-week wash-out/dietary lead-in period and then were randomized to a 12 week treatment with either pitavastatin tablets or simvastatin (Table 6). Non-inferiority of pitavastatin to a given dose of simvastatin was considered to be demonstrated if the lower bound of the 95% CI for the mean treatment difference was greater than -6% for the mean percent change in LDL-C.
Lipid results are shown in Table 6. For the percent change from baseline to endpoint in LDL-C, pitavastatin tablets was non-inferior to simvastatin for the two pairwise comparisons: Pitavastatin tablets 2 mg vs. simvastatin 20 mg and pitavastatin tablets 4 mg vs. simvastatin 40 mg. Mean treatment differences (95% CI) were 4% (1%, 7%) and 1% (-2%, 4%), respectively.
| Treatment | N | LDL-C | Apo-B | TC | TG | HDL-C | non-HDL-C |
| Pitavastatin Tablets 2 mg daily | 307 | -39 | -30 | -28 | -16 | 6 | -36 |
| Pitavastatin Tablets 4 mg daily | 319 | -44 | -35 | -32 | -17 | 6 | -41 |
| Simvastatin 20 mg daily | 107 | -35 | -27 | -25 | -16 | 6 | -32 |
| Simvastatin 40 mg daily | 110 | -43 | -34 | -31 | -16 | 7 | -39 |
| Simvastatin 80 mg | ---------------------------------------Not Studied------------------------------------------------------------ | ||||||
Active-controlled study with pravastatin in elderly (NK-104-306): Pitavastatin tablets was compared with the HMG-CoA reductase inhibitor pravastatin in a randomized, multicenter, double-blind, double-dummy, parallel group, active-controlled non-inferiority Phase 3 study of 942 elderly patients (≥65 years) with primary hyperlipidemia or mixed dyslipidemia. Patients entered a 6- to 8-week wash-out/dietary lead-in period, and then were randomized to a once daily dose of pitavastatin tablets or pravastatin for 12 weeks (Table 7). Non-inferiority of pitavastatin tablets to a given dose of pravastatin was assumed if the lower bound of the 95% CI for the treatment difference was greater than -6% for the mean percent change in LDL-C.
Lipid results are shown in Table 7. Pitavastatin tablets significantly reduced LDL-C compared to pravastatin as demonstrated by the following pairwise dose comparisons: Pitavastatin tablets 1 mg vs. pravastatin 10 mg, pitavastatin tablets 2 mg vs. pravastatin 20 mg and pitavastatin tablets 4 mg vs. pravastatin 40 mg. Mean treatment differences (95% CI) were 9% (6%, 12%), 10% (7%, 13%) and 10% (7%, 13% ), respectively.
| Treatment | N | LDL-C | Apo-B | TC | TG | HDL-C | non-HDL-C |
| Pitavastatin Tablets 1 mg daily | 207 | -31 | -25 | -22 | -13 | 1 | -29 |
| Pitavastatin Tablets 2 mg daily | 224 | -39 | -31 | -27 | -15 | 2 | -36 |
| Pitavastatin Tablets 4 mg daily | 210 | -44 | -37 | -31 | -22 | 4 | -41 |
| Pravastatin 10 mg daily | 103 | -22 | -17 | -15 | -5 | 0 | -20 |
| Pravastatin 20 mg daily | 96 | -29 | -22 | -21 | -11 | -1 | -27 |
| Pravastatin 40 mg daily | 102 | -34 | -28 | -24 | -15 | 1 | -32 |
| Pravastatin 80 mg daily |
--------------------------------------Not Studied------------------------------------------------------------ |
||||||
Active-controlled study with simvastatin in patients with ≥ 2 risk factors for coronary heart disease (NK-104-304): Pitavastatin tablets was compared with the HMG-CoA reductase inhibitor simvastatin in a randomized, multicenter, double-blind, double-dummy, active-controlled, non-inferiority Phase 3 study of 351 patients with primary hyperlipidemia or mixed dyslipidemia with ≥2 risk factors for coronary heart disease. After a 6- to 8-week wash-out/dietary lead-in period, patients were randomized to a 12-week treatment with either pitavastatin tablets or simvastatin (Table 8). Non-inferiority of pitavastatin tablets to simvastatin was considered to be demonstrated if the lower bound of the 95% CI for the mean treatment difference was greater than -6% for the mean percent change in LDL-C.
Lipid results are shown in Table 8. Pitavastatin tablets 4 mg was non-inferior to simvastatin 40 mg for percent change from baseline to endpoint in LDL-C. The mean treatment difference (95% CI) was 0% (-2%, 3%).
| Treatment | N | LDL-C | Apo-B | TC | TG | HDL-C | non-HDL-C |
| Pitavastatin Tablets 4 mg daily | 233 | -44 | -34 | -31 | -20 | 7 | -40 |
| Simvastatin 40 mg daily | 118 | -44 | -34 | -31 | -15 | 5 | -39 |
| Simvastatin 80 mg daily |
--------------------------------------Not Studied-------------------------------------------------------------- |
||||||
Active-controlled study with atorvastatin in patients with type II diabetes mellitus (NK-104-305): Pitavastatin tablets was compared with the HMG-CoA reductase inhibitor atorvastatin in a randomized, multicenter, double-blind, double-dummy, parallel group, active-controlled, non-inferiority Phase 3 study of 410 subjects with type II diabetes mellitus and combined dyslipidemia. Patients entered a 6- to 8-week washout/dietary lead-in period and were randomized to a once daily dose of pitavastatin tablets or atorvastatin for 12 weeks. Non-inferiority of pitavastatin tablets was considered to be demonstrated if the lower bound of the 95% CI for the mean treatment difference was greater than -6% for the mean percent change in LDL-C.
Lipid results are shown in Table 9. The treatment difference (95% CI) for LDL-C percent change from baseline was -2% (-6.2%, 1.5%). The two treatment groups were not statistically different on LDL-C. However, the lower limit of the CI was -6.2%, slightly exceeding the -6% non-inferiority limit so that the non-inferiority objective was not achieved.
| Treatment | N | LDL-C | Apo-B | TC | TG | HDL-C | non-HDL-C |
| Pitavastatin Tablets 4 mg daily | 274 | -41 | -32 | -28 | -20 | 7 | -36 |
| Atorvastatin 20 mg daily | 136 | -43 | -34 | -32 | -27 | 8 | -40 |
| Atorvastatin 40 mg daily | -----------------------------------Not Studied---------------------------------------------------- | ||||||
| Atorvastatin 80 mg daily | -----------------------------------Not Studied----------------------------------------------------- | ||||||
The treatment differences in efficacy in LDL-C change from baseline between pitavastatin tablets and active controls in the Phase 3 studies are summarized in Figure 1.
Figure 1. Treatment Difference in Adjusted Mean Percent Change in LDL-C
16 HOW SUPPLIED/STORAGE AND HANDLING
Pitavastatin tablets for oral administration are provided as white to off-white, film-coated tablets that contain 1 mg, 2 mg, or 4 mg of pitavastatin.
Packaging
Pitavastatin tablets, 1 mg are round white to off-white film-coated tablets debossed with "P1" on one side.
Bottle of 90 tablets NDC: 69076-030-11
Pitavastatin tablets, 2 mg are round white to off-white film-coated tablets debossed with "P2" on one side.
Bottle of 90 tablets NDC: 69076-031-11
Pitavastatin tablets, 4 mg are round white to off-white film-coated tablets debossed with "P4" on one side.
Bottle of 90 tablets NDC: 69076-032-11
17 PATIENT COUNSELING INFORMATION
The patient should be informed of the following:
Dosing Time
Pitavastatin tablets can be taken at any time of the day with or without food.
Muscle Pain
Patients should be advised to promptly notify their physician of any unexplained muscle pain,tenderness, or weakness particularly if accompanied by malaise or fever, or if these muscle signs orsymptoms persist after discontinuing pitavastatin tablets. They should discuss all medication, both prescriptionand over the counter, with their physician.
Embryo-fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus, to use effective contraceptionduring treatment and to inform their healthcare professional of a known or suspected pregnancy [seeContraindications (4), Use in Specific Populations (8.1, 8.3)].
Lactation
Advise women not to breastfeed during treatment with pitavastatin tablets [see Contraindications (4), Use inSpecific Populations (8.2)].
Liver Enzymes
It is recommended that liver enzyme tests be checked before the initiation of pitavastatin tablets and if signs or symptoms of liver injury occur. All patients treated with pitavastatin tablets should be advised to report promptly any symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice.
Manufactured by: Standard Chem & Pharm Co., Ltd, Tainan City, Taiwan 73055 for Quinn Pharmaceuticals, LLC, Coral Springs. FL 33067
To request additional information or if you have questions concerning pitavastatin tablets please phone Quinn Pharmaceuticals, LLC at 800-244-0277.
PRINCIPAL DISPLAY PANEL- Pitavastatin 1 mg
NDC: 69076-030-11
Pitavastatin
Tablets
1 mg
Rx Only
Manufactured by:
Standard Chem & Pharm Co.,
Made in Taiwan
Manufactured for:
Quinn Pharmaceuticals, LLC
Coral Springs. FL

PRINCIPAL DISPLAY PANEL - Pitavastatin 2 mg
NDC: 69076-031-11
Pitavastatin
Tablets
2 mg
Rx Only
Manufactured by:
Standard Chem & Pharm Co.,
Made in Taiwan
Manufactured for:
Quinn Pharmaceuticals, LLC
Coral Springs. FL

PRINCIPAL DISPLAY PANEL- Pitavastatin 4 mg
NDC: 69076-032-11
Pitavastatin
Tablets
4 mg
Rx Only
Manufactured by:
Standard Chem & Pharm Co.,
Made in Taiwan
Manufactured for:
Quinn Pharmaceuticals, LLC
Coral Springs. FL

| PITAVASTATIN
pitavastatin calcium tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| PITAVASTATIN
pitavastatin calcium tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| PITAVASTATIN
pitavastatin calcium tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - QUINN PHARMACEUTICALS, LLC (079421782) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Standard Chem & Pharm Co, Ltd | 871402375 | MANUFACTURE(69076-030, 69076-031, 69076-032) | |