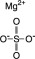SUPREP BOWEL PREP- sodium sulfate, potassium sulfate, magnesium sulfate solution, concentrate
SUPREP Bowel Prep by
Drug Labeling and Warnings
SUPREP Bowel Prep by is a Prescription medication manufactured, distributed, or labeled by Braintree Laboratories, Inc., BrainTree Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SUPREP Bowel Prep Kit safely and effectively. See full prescribing information for SUPREP Bowel Prep Kit
SUPREP Bowel Prep Kit (sodium sulfate, potassium sulfate, and magnesium sulfate) Oral Solution
Initial U.S. Approval: 08/2010RECENT MAJOR CHANGES
Dosage and Administration 11/2012 INDICATIONS AND USAGE
SUPREP Bowel Prep Kit is an osmotic laxative indicated for cleansing of the colon in preparation for colonoscopy in adults (1)
DOSAGE AND ADMINISTRATION
Dilute the solution prior to use. See FULL PRESCRIBING INFORMATION for complete dosing and administration instructions (2)
Split Dose (2-Day) Regimen
- Evening before colonoscopy: dilute one bottle with water to a total volume of 16 ounces (up to the fill line) and drink the entire amount.
- Drink 32 ounces of water over the next hour.
- Next morning: repeat both steps using the second bottle.
- Complete preparation at least 2 hours before colonoscopy or as directed by physician.
DOSAGE FORMS AND STRENGTHS
- Two 6 ounce bottles of oral solution, each containing sodium sulfate 17.5 grams, potassium sulfate 3.13 grams, and magnesium sulfate 1.6 grams. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Risk of fluid and electrolyte abnormalities, arrhythmias, seizures and renal impairment – assess concurrent medications and consider testing in some patients (5.1, 5.2, 5.3)
- Patients with renal insufficiency – use caution, ensure adequate hydration and consider testing (5.4)
- Suspected GI obstruction or perforation – rule out the diagnosis before administration (4, 5.6)
- Patients at risk for aspiration – observe during administration (5.7)
- Not for direct ingestion – dilute and take with additional water (5.8)
ADVERSE REACTIONS
Most common adverse reactions (≥3%) are: overall discomfort, abdominal fullness, nausea, abdominal cramping, and vomiting (6)
To report SUSPECTED ADVERSE REACTIONS, contact Braintree Laboratories, Inc. at 1-800-874-6756 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Fluid and Serum Chemistry Abnormalities
5.2 Cardiac Arrhythmias
5.3 Seizures
5.4 Renal Impairment
5.5 Colonic Mucosal Ulcerations and Ischemic Colitis
5.6 Use in Patients with Significant Gastrointestinal Disease
5.7 Aspiration
5.8 Not for Direct Ingestion
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
7 DRUG INTERACTIONS
7.1 Drugs That May Increase Risks Due to Fluid and Electrolyte Abnormalities
7.2 Potential for Altered Drug Absorption
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Patient Counseling
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
SUPREP Bowel Prep Kit should be taken as a split-dose oral regimen.
The dose for colon cleansing requires administration of two bottles of SUPREP Bowel Prep Kit. Each bottle is administered as 16 ounces of diluted SUPREP solution with an additional 1 quart of water taken orally. The total volume of liquid required for colon cleansing (using two bottles) is 3 quarts (approximately 2.8 L) taken orally prior to the colonoscopy in the following way: Split-Dose (Two-Day) Regimen
Day prior to colonoscopy:
- A light breakfast may be consumed, or have only clear liquids on the day before colonoscopy. Avoid red and purple liquids, milk, and alcoholic beverages.
- Early in the evening prior to colonoscopy: pour the contents of one bottle of SUPREP Bowel Prep Kit into the mixing container provided. Fill the container with water to the 16 ounce fill line, and drink the entire amount.
- Drink two additional containers filled to the 16 ounce line with water over the next hour.
Day of colonoscopy:
- Have only clear liquids until after the colonoscopy. Avoid red and purple liquids, milk, and alcoholic beverages.
- The morning of colonoscopy (10 to 12 hours after the evening dose): pour the contents of the second bottle of SUPREP Bowel Prep Kit into the mixing container provided. Fill the container with water to the 16 ounce fill line, and drink the entire amount.
- Drink two additional containers filled to the 16 ounce line with water over the next hour.
- Complete all SUPREP Bowel Prep Kit and required water at least two hours prior to colonoscopy or as directed by physician.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- Gastrointestinal obstruction
- Bowel perforation
- Gastric retention
- Ileus
- Toxic colitis or toxic megacolon
- Known allergies to components of the kit [see Description (11)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Fluid and Serum Chemistry Abnormalities
Advise all patients to hydrate adequately before, during, and after the use of SUPREP Bowel Prep Kit. If a patient develops significant vomiting or signs of dehydration after taking SUPREP Bowel Prep Kit, consider performing post-colonoscopy lab tests (electrolytes, creatinine, and BUN). Fluid and electrolyte disturbances can lead to serious adverse events including cardiac arrhythmias, seizures and renal impairment.
Patients with electrolyte abnormalities should have them corrected before treatment with SUPREP Bowel Prep Kit. In addition, use caution when prescribing SUPREP Bowel Prep Kit for patients with conditions, or who are using medications, that increase the risk for fluid and electrolyte disturbances or may increase the risk of adverse events of seizure, arrhythmias, and renal impairment. [see Drug Interactions (7.1)]
SUPREP Bowel Prep Kit can cause temporary elevations in uric acid. [see Adverse Reactions (6.1)]. Uric acid fluctuations in patients with gout may precipitate an acute flare. The potential for uric acid elevation should be considered before administering SUPREP Bowel Prep Kit to patients with gout or other disorders of uric acid metabolism.
5.2 Cardiac Arrhythmias
There have been rare reports of serious arrhythmias associated with the use of ionic osmotic laxative products for bowel preparation. Use caution when prescribing SUPREP Bowel Prep Kit for patients at increased risk of arrhythmias (e.g., patients with a history of prolonged QT, uncontrolled arrhythmias, recent myocardial infarction, unstable angina, congestive heart failure, or cardiomyopathy). Pre-dose and post-colonoscopy ECGs should be considered in patients at increased risk of serious cardiac arrhythmias.
5.3 Seizures
There have been reports of generalized tonic-clonic seizures and/or loss of consciousness associated with use of bowel preparation products in patients with no prior history of seizures. The seizure cases were associated with electrolyte abnormalities (e.g., hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia) and low serum osmolality. The neurologic abnormalities resolved with correction of fluid and electrolyte abnormalities.
Use caution when prescribing SUPREP Bowel Prep Kit for patients with a history of seizures and in patients at increased risk of seizure, such as patients taking medications that lower the seizure threshold (e.g., tricyclic antidepressants), patients withdrawing from alcohol or benzodiazepines, or patients with known or suspected hyponatremia.
5.4 Renal Impairment
Use caution when prescribing SUPREP Bowel Prep Kit for patients with impaired renal function or patients taking concomitant medications that may affect renal function (such as diuretics, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or non-steroidal anti-inflammatory drugs). Advise these patients of the importance of adequate hydration, and consider performing baseline and post-colonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients.
5.5 Colonic Mucosal Ulcerations and Ischemic Colitis
Administration of osmotic laxative products may produce colonic mucosal aphthous ulcerations, and there have been reports of more serious cases of ischemic colitis requiring hospitalization. Concurrent use of stimulant laxatives and SUPREP Bowel Prep Kit may increase these risks. The potential for mucosal ulcerations resulting from the bowel preparation should be considered when interpreting colonoscopy findings in patients with known or suspect inflammatory bowel disease (IBD).
5.6 Use in Patients with Significant Gastrointestinal Disease
If gastrointestinal obstruction or perforation is suspected, perform appropriate diagnostic studies to rule out these conditions before administering SUPREP Bowel Prep Kit.
Use with caution in patients with severe active ulcerative colitis.
5.7 Aspiration
Use with caution in patients with impaired gag reflex and patients prone to regurgitation or aspiration. Such patients should be observed during administration of SUPREP Bowel Prep Kit solution.
5.8 Not for Direct Ingestion
Each bottle must be diluted with water to a final volume of 16 ounces and ingestion of additional water as recommended is important to patient tolerance. Direct ingestion of the undiluted solution may increase the risk of nausea, vomiting, dehydration, and electrolyte disturbances.
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in clinical studies of another drug and may not reflect the rates observed in practice.
In a multicenter, controlled clinical trial comparing SUPREP Bowel Prep Kit with a bowel prep containing polyethylene glycol and electrolytes (PEG + E) that were administered in a split-dose (2-day) regimen, the most common adverse reactions after administration of SUPREP Bowel Prep Kit were overall discomfort, abdominal distention, abdominal pain, nausea, vomiting, and headache; see Table 1, below. Less common Adverse Reactions occurring were AV Block (1 case) and CK increase. In this study, patients receiving SUPREP Bowel Prep Kit were limited to a light breakfast followed by clear liquids; patients receiving the PEG + E bowel prep were allowed to have a normal breakfast and a light lunch, followed by clear liquids.
Table 1: Treatment-Emergent Adverse Reactions Observed in at Least 2% of Patients on the Split-Dose (2-Day) Regimen Symptom Split-Dose (2-Day) Regimen SUPREP
N=190PEG + E product
N=189Overall Discomfort 54% 67% Abdominal Distension 40% 52% Abdominal Pain 36% 43% Nausea 36% 33% Vomiting 8% 4% Headache 1.1% 0.5% Table 2 shows the percentages of patients who developed new abnormalities of important electrolytes and uric acid after completing the bowel preparation with either SUPREP Bowel Prep Kit or PEG+E administered as a split-dose (2-day) regimen.
Table 2: Patients with Normal Baseline Serum Chemistry with A Shift to an Abnormal Value While on the Split-Dose (2-Day) Regimen *Percent (n/N) of patients where N=number of patients with normal baseline who had abnormal values at the timepoint(s) of interest.
†Patients with normal bicarbonate at baseline who developed low bicarbonate (≤ 21 mEq/L) and high anion gap (≥ 13 mEq/L) on Day of Colonoscopy or Day 30.
Day of Colonoscopy n (%)* Day 30 n (%)* Anion gap (high) † SUPREP 14 (8.9) 3 (1.9) PEG + Electrolytes 12 (7.6) 2 (1.4) Bicarbonate (low) SUPREP 20 (12.7) 7 (4.4) PEG + Electrolytes 24 (15.2) 4 (2.7) Bilirubin, total (high) SUPREP 14 (8.5) 0 (0) PEG + Electrolytes 20 (11.7) 3 (1.9) BUN (high) SUPREP 2 (1.6) 14 (11.2) PEG + Electrolytes 4 (2.9) 19 (14.5) Calcium (high) SUPREP 16 (10.4) 8 (5.2) PEG + Electrolytes 6 (3.7) 6 (3.9) Chloride (high) SUPREP 4 (2.4) 6 (3.7) PEG + Electrolytes 20 (12.2) 6 (3.8) Creatinine (high) SUPREP 3 (1.9) 5 (3.2) PEG + Electrolytes 2 (1.2) 8 (5.2) Osmolality (high) SUPREP 8 (5.8) NA PEG + Electrolytes 19 (12.9) NA Osmolality (low) SUPREP 3 (2.2) NA PEG + Electrolytes 2 (1.4) NA Potassium (high) SUPREP 3 (1.8) 6 (3.7) PEG + Electrolytes 5 (2.9) 8 (4.9) Sodium (low) SUPREP 5 (3.1) 1 (0.6) PEG + Electrolytes 4 (2.3) 2 (1.2) Uric acid (high) SUPREP 27 (23.5) 13 (11.5) PEG + Electrolytes 12 (9.5) 20 (16.7) There were also 408 patients who participated in a study in which either SUPREP Bowel Prep Kit or PEG+E were administered in an evening-only (1-day) regimen. Higher rates of overall discomfort, abdominal distention, and nausea were observed with the evening-only (1-day) regimen compared to the split-dose (2-day) regimen for both preparations. Patients treated with SUPREP Bowel Prep Kit had increased rates of vomiting with the evening-only (1-day) regimen. An evening-only (1-day) dosing regimen was associated with higher rates of abnormal values for some electrolytes when compared to the split-dose (2-day) regimen for both preparations. For SUPREP Bowel Prep Kit, the evening-only (1-day) regimen was associated with higher rates of total bilirubin (high), BUN (high), creatinine (high), osmolality (high), potassium (high) and uric acid (high) than the SUPREP Bowel Prep Kit split-dose (2day) regimen. Administration of SUPREP Bowel Prep Kit in an evening-only (1-day) dosing regimen is not recommended.
-
7 DRUG INTERACTIONS
7.1 Drugs That May Increase Risks Due to Fluid and Electrolyte Abnormalities
Use caution when prescribing SUPREP Bowel Prep Kit for patients with conditions, or who are using medications, that increase the risk for fluid and electrolyte disturbances or may increase the risk of adverse events of seizure, arrhythmias, and prolonged QT in the setting of fluid and electrolyte abnormalities. Consider additional patient evaluations as appropriate [see Warnings (5)] in patients taking these concomitant medications.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic effects: Pregnancy Category C. Animal reproduction studies have not been conducted with SUPREP Bowel Prep Kit. It is also not known whether SUPREP Bowel Prep Kit can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. SUPREP Bowel Prep Kit should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when SUPREP Bowel Prep Kit is administered to a nursing woman.
8.5 Geriatric Use
Of the 375 patients who received SUPREP Bowel Prep Kit in clinical trials, 94 (25%) were 65 years of age or older, and 25 (7%) were 75 years of age or older. No overall differences in safety or effectiveness of SUPREP Bowel Prep Kit administered as a split-dose (2-day) regimen were observed between geriatric patients and younger patients. Geriatric patients reported more vomiting when SUPREP Bowel Prep Kit was given as a one-day preparation.
-
11 DESCRIPTION
Each SUPREP Bowel Prep Kit contains two 6 ounce bottles of solution. Each 6 ounce bottle contains: sodium sulfate
17.5 grams, potassium sulfate 3.13 grams, magnesium sulfate 1.6 grams. Inactive ingredients include: sodium benzoate, NF, sucralose, malic acid FCC, citric acid USP, flavoring ingredients, purified water, USP. The solution is a clear to slightly hazy liquid. The solution is clear and colorless when diluted to a final volume of 16 ounces with water.
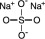
Sodium Sulfate, USP
The chemical name is Na2SO4. The average Molecular Weight is 142.04. The structural formula is:
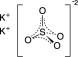
Potassium Sulfate, FCC, purified
The chemical name is K2SO4. The average Molecular Weight is 174.26. The structural formula is:
Magnesium Sulfate, USP
The chemical name is MgSO4. The average Molecular Weight: 120.37. The structural formula is:
Each SUPREP Bowel Prep Kit also contains a polypropylene mixing container.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Sulfate salts provide sulfate anions, which are poorly absorbed. The osmotic effect of unabsorbed sulfate anions and the associated cations causes water to be retained within the gastrointestinal tract.
12.2 Pharmacodynamics
The osmotic effect of the unabsorbed ions, when ingested with a large volume of water, produces a copious watery diarrhea.
12.3 Pharmacokinetics
Fecal excretion was the primary route of sulfate elimination. After administration of SUPREP Bowel Prep Kit in six healthy volunteers, the time at which serum sulfate reached its highest point (Tmax) was approximately 17 hours after the first half dose or approximately 5 hours after the second dose, and then declined with a half-life of 8.5 hours.
The disposition of sulfate after SUPREP Bowel Prep Kit was also studied in patients (N=6) with mild-moderate hepatic impairment (Child-Pugh grades A and B) and in patients (N=6) with moderate renal impairment (creatinine clearance of 30 to 49 mL/min). The renal impairment group had the highest serum sulfate AUC and Cmax, followed by the hepatic impairment group, and then by healthy subjects. Systemic exposure of serum sulfate (AUC and Cmax) was similar between healthy subjects and hepatic impairment patients. Renal impairment resulted in 54% higher mean AUC and 44% higher mean Cmax than healthy subjects. The mean sulfate levels of all three groups returned to their respective baseline levels by Day 6 after dose initiation. Urinary excretion of sulfate over 30 hours, starting after the first half dose, was similar between hepatic patients and normal volunteers, but was approximately 16% lower in moderate renal impairment patients than in healthy volunteers.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of SUPREP Bowel Prep Kit. Studies to evaluate the possible impairment of fertility or mutagenic potential of SUPREP Bowel Prep Kit have not been performed.
13.2 Animal Toxicology and/or Pharmacology
The sulfate salts of sodium, potassium, and magnesium contained in SUPREP Bowel Prep Kit were administered orally (gavage) to rats and dogs up to 28 days up to a maximum daily dose of 5 grams/kg/day (approximately 0.9 and 3 times for rats and dogs, respectively, the recommended human dose of 44 grams/day or 0.89 grams/kg based on the body surface area). In rats, the sulfate salts caused diarrhea and electrolyte and metabolic changes, including hypochloremia, hypokalemia, hyponatremia, lower serum osmolality, and high serum bicarbonate. Significant renal changes included increased fractional sodium excretion, increased urinary sodium and potassium excretion, and alkaline urine in both males and females. In addition, creatinine clearance was significantly decreased in females at the highest dose. No microscopic renal changes were seen. In dogs, the sulfate salts caused emesis, excessive salivation, excessive drinking of water, and abnormal excreta (soft and/or mucoid feces and/or diarrhea) and increased urine pH and sodium excretion.
-
14 CLINICAL STUDIES
The colon cleansing efficacy of SUPREP Bowel Prep Kit was evaluated in a randomized, single-blind, active-controlled, multicenter study. In this study, 363 adult patients were included in the efficacy analysis. Patients ranged in age from 20 to 84 years (mean age 55 years) and 54% were female. Race distribution was 86% Caucasian, 9% African-American, and 5% other.
Patients were randomized to one of the following two colon preparation regimens: SUPREP Bowel Prep Kit or a marketed polyethylene glycol (PEG) bowel prep. In the Study SUPREP Bowel Prep Kit was administered according to a split-dose preparation regimen [see Dosage and Administration (2.1)]. The PEG bowel prep was also given as a split-dose preparation according to its labeled instructions. Patients receiving SUPREP Bowel Prep Kit were limited to a light breakfast followed by clear liquids on the day prior to the day of colonoscopy; patients receiving the PEG bowel prep were allowed to have a normal breakfast and a light lunch, followed by clear liquids.
The primary efficacy endpoint was the proportion of patients with successful colon cleansing as assessed by the colonoscopists, who were not informed about the type of preparation received. In the study, no clinically or statistically significant differences were seen between the group treated with SUPREP Bowel Prep Kit and the group treated with the PEG bowel prep.
See Table 3 below.
Table 3: Colon Cleansing Response Rates 1 Responders were patients whose colon preparations were graded excellent (no more than small bits of adherent feces/fluid) or good (small amounts of feces or fluid not interfering with the exam) by the colonoscopist.
2 Does not equal difference in tabled responder rates due to rounding effects.
Treatment Group Regimen N Responders1
%
(95% C. I.)SUPREP – PEG
Difference
(95% CI)SUPREP Bowel Prep Kit
(with light breakfast)Split-Dose 180 97%
(94%, 99%)2%2 (-2%, 5%) PEG bowel prep
(with normal breakfast & light lunch)Split-Dose 183 96%
(92%, 98%) -
16 HOW SUPPLIED/STORAGE AND HANDLING
Each SUPREP Bowel Prep Kit contains:
- Two (2) 6 ounce bottles of oral solution.
- One (1) 19 ounce mixing container with a 16 ounce fill line.
Storage:
Store at 20° to 25°C (68° to 77°F). Excursions permitted between 15° to 30°C (59° to 86°F). See USP controlled room temperature.
Keep out of reach of children.
SUPREP Bowel Prep Kit NDC: 52268-012-01
-
17 PATIENT COUNSELING INFORMATION
See Medication Guide and FDA-Approved Patient Labeling
17.1 Patient Counseling
- Ask patients to let you know if they have trouble swallowing or are prone to regurgitation or aspiration.
- Instruct patients that each bottle needs to be diluted in water before ingestion and that they need to drink additional water according to the instructions. Direct ingestion of the undiluted solution may increase the risk of nausea, vomiting, and dehydration.
- Inform patients that oral medications may not be absorbed properly if they are taken within one hour of starting each dose of SUPREP Bowel Prep Kit.
- Tell patients not to take other laxatives while they are taking SUPREP Bowel Prep Kit.
Distributed by Braintree Laboratories, Inc.
Braintree, MA 02185
U.S. Patent 6,946,149 -
MEDICATION GUIDE
Medication Guide
SUPREP®(Soo-prep) Bowel Prep Kit
(sodium sulfate, potassium sulfate and magnesium sulfate) Oral Solution
Read this Medication Guide before you start taking SUPREP Bowel Prep Kit. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about SUPREP Bowel Prep Kit?
SUPREP Bowel Prep Kit and other osmotic bowel preparations can cause serious side effects, including:
Serious loss of body fluid (dehydration) and changes in blood salts (electrolytes) in your blood.
These changes can cause:
- abnormal heartbeats that can cause death
- seizures. This can happen even if you have never had a seizure.
- kidney problems
Your chance of having fluid loss and changes in body salts with SUPREP Bowel Prep Kit is higher if you:
- have heart problems
- have kidney problems
- take water pills or non-steroidal anti-inflammatory drugs (NSAIDS)
Tell your healthcare provider right away if you have any of these symptoms of a loss of too much body fluid (dehydration) while taking SUPREP Bowel Prep Kit:
- vomiting that prevents you from keeping down the additional prescribed amount of water listed in the Instructions for Use in the Patient Instructions for Use Booklet
- dizziness
- urinating less often than normal
- headache
See Section “What are the possible side effects of SUPREP Bowel Prep Kit?” for more information about side effects.
What is SUPREP Bowel Prep Kit?
SUPREP Bowel Prep Kit is a prescription medicine used by adults to clean the colon before a colonoscopy. SUPREP Bowel Prep Kit cleans your colon by causing you to have diarrhea. Cleaning your colon helps your healthcare provider see the inside of your colon more clearly during your colonoscopy.
It is not known if SUPREP Bowel Prep Kit is safe and effective in children.
Who should not take SUPREP Bowel Prep Kit?
Do not take SUPREP Bowel Prep Kit if your heathcare provider has told you that you have:
- a blockage in your bowel (obstruction)
- an opening in the wall of your stomach or intestine (bowel perforation)
- problems with food and fluid emptying from your stomach (gastric retention)
- a very dilated intestine (bowel)
- an allergy to any of the ingredients in SUPREP Bowel Prep Kit. See the end of this leaflet for a complete list of ingredients in SUPREP Bowel Prep Kit.
What should I tell my healthcare provider before taking SUPREP Bowel Prep Kit?
Before you take SUPREP Bowel Prep Kit, tell your healthcare provider if you:
- have heart problems
- have stomach or bowel problems
- have ulcerative colitis
- have problems with swallowing or gastric reflux
- have gout
- have a history of seizures
- are withdrawing from drinking alcohol
- have a low blood salt (sodium) level
- have kidney problems
- any other medical conditions
- are pregnant. It is not known if SUPREP Bowel Prep Kit will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if SUPREP Bowel Prep Kit passes into your breast milk. You and your healthcare provider should decide if you will take SUPREP Bowel Prep Kit while breastfeeding.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
SUPREP Bowel Prep Kit may affect how other medicines work. Medicines taken by mouth may not be absorbed properly when taken within 1 hour before the start of each dose of SUPREP Bowel Prep Kit.
Especially tell your healthcare provider if you take:
- medicines for blood pressure or heart problems
- medicines for kidney problems
- medicines for seizures
- water pills (diuretics)
- non-steroidal anti-inflammatory medicines (NSAID) pain medicines
- laxatives
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure if you are taking any of the medicines listed above.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take SUPREP Bowel Prep Kit?
See the Instructions for Use in the Patient Instructions for Use Booklet for dosing instructions. You must read, understand, and follow these instructions to take SUPREP Bowel Prep Kit the right way.
- Take SUPREP Bowel Prep Kit exactly as your healthcare provider tells you to take it
- Do not drink SUPREP Bowel Prep Kit solution that has not been mixed with water (diluted), it may increase your risk of nausea, vomiting and fluid loss (dehydration).
- Each bottle of SUPREP Bowel Prep Kit must be mixed with water (diluted) before drinking.
- It is important for you to drink the additional prescribed amount of water listed in the Instructions for Use to prevent fluid loss (dehydration).
- Do not take other laxatives while taking SUPREP Bowel Prep Kit.
- Do not eat solid foods while taking SUPREP Bowel Prep Kit. Only clear liquids are allowed while taking SUPREP Bowel Prep Kit.
What are the possible side effects of SUPREP Bowel Prep Kit?
SUPREP Bowel Prep Kit can cause serious side effects, including:
- See Section “What is the most important information I should know about SUPREP Bowel Prep Kit?”
- changes in certain blood tests. Your healthcare provider may do blood tests after you take SUPREP Bowel Prep Kit to check your blood for changes. Tell your healthcare provider if you have any symptoms of too much fluid loss, including:
- vomiting nausea
- bloating
- dizziness
- stomach (abdominal) cramping
- headache
- urinate less than usual
- trouble drinking clear liquid
- heart problems. SUPREP Bowel Prep Kit may cause irregular heartbeats.
- seizures
- ulcers of the bowel or bowel problems
- worsening gout
The most common side effects of SUPREP Bowel Prep Kit include:
- discomfort
- bloating
- stomach (abdominal) cramping
- nausea
- vomiting
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of SUPREP Bowel Prep Kit. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800FDA-1088.
How should I store SUPREP Bowel Prep Kit?
- Store SUPREP Bowel Prep Kit at room temperature, between 59°F to 86°F (15°C to 30°C).
Keep SUPREP Bowel Prep Kit and all medicines out of the reach of children.
General information about the safe and effective use of SUPREP Bowel Prep Kit.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use SUPREP Bowel Prep Kit for a condition for which it was not prescribed. Do not give SUPREP Bowel Prep Kit to other people, even if they are going to have the same procedure you are. It may harm them.
This Medication Guide summarizes important information about SUPREP Bowel Prep Kit. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information that is written for healthcare professionals.
For more information, go to www.braintreelabs.com or call 1-800-874-6756.
What are the ingredients in SUPREP Bowel Prep Kit?
Active ingredients: sodium sulfate, potassium sulfate and magnesium sulfate
Inactive ingredients: sodium benzoate, sucralose, malic acid, citric acid, flavoring ingredients, purified water
Braintree Laboratories, Inc
Braintree, MA 02185, USAThis Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised 01/2017
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Carton Label
NDC: 52268-012-01 U.S. Patent 6,946,149
Dispense the enclosed Medication Guide to each patient.
SUPREP®
BOWEL PREP KIT
(sodium sulfate, potassium
sulfate and magnesium sulfate)
Oral Solution(17.5g/3.13g/1.6g) per 6 ounces
This carton contains:
2 6-ounce (177 mL) bottles of liquid bowel prep
1 16-ounce mixing container
1 Patient booklet. Booklet includes:
1- Medication Guide
2- Patient Instructions
3- Full Prescribing InformationDilute the solution concentrate as
directed prior to use.Both 6-ounce bottles are required for
a complete prep.Store at 25°C (77°F): excursions
permitted to 15-30°C (59-86°F).RX only
LABORATORIES INC
Braintree
-
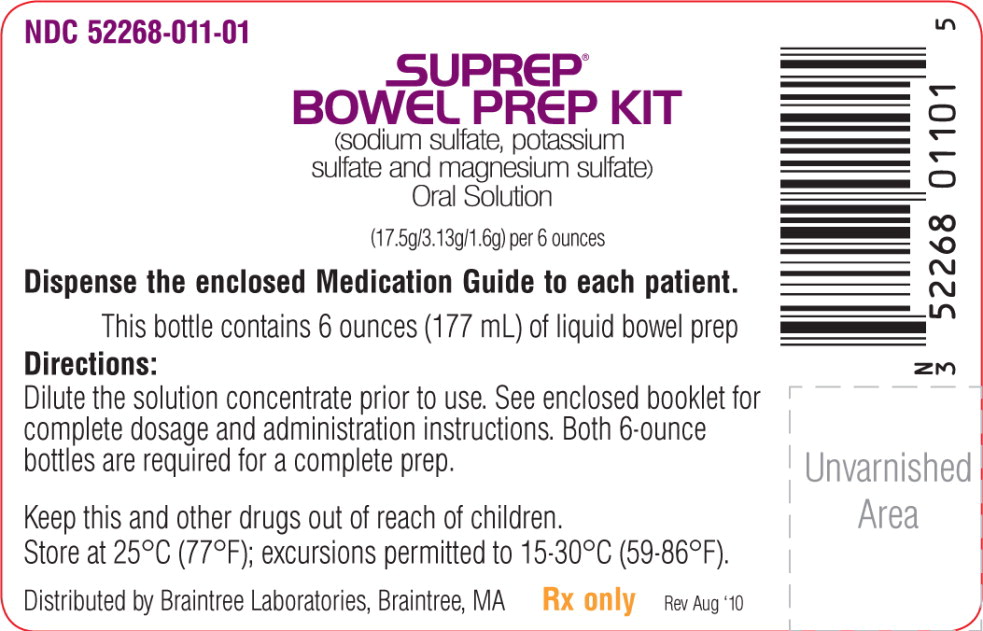
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Bottle Label
NDC: 52268-011-01
SUPREP®
BOWEL PREP KIT
(sodium sulfate, potassium
sulfate and magnesium sulfate)
Oral Solution(17.5g/3.13g/1.6g) per 6 ounces
Dispense the enclosed Medication Guide to each patient.
This bottle contains 6 ounces (177 mL) of liquid bowel prep
Directions:
Dilute the solution concentrate prior to use. See enclosed booklet for
complete dosage and administration instructions. Both 6-ounce
bottles are required for a complete prep.Keep this and other drugs out of reach of children.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).Distributed by Braintree Laboratories, Braintree, MA Rx only Rev Aug ‘10
-
INGREDIENTS AND APPEARANCE
SUPREP BOWEL PREP
sodium sulfate, potassium sulfate, magnesium sulfate solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52268-012 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sodium sulfate (UNII: 0YPR65R21J) (sodium cation - UNII:LYR4M0NH37) sodium sulfate 17.5 g in 1 mL potassium sulfate (UNII: 1K573LC5TV) (potassium cation - UNII:295O53K152) potassium sulfate 3.13 g in 1 mL magnesium sulfate, unspecified form (UNII: DE08037SAB) (magnesium cation - UNII:T6V3LHY838) magnesium sulfate, unspecified form 1.6 g in 1 mL Inactive Ingredients Ingredient Name Strength sodium benzoate (UNII: OJ245FE5EU) sucralose (UNII: 96K6UQ3ZD4) malic acid (UNII: 817L1N4CKP) citric acid monohydrate (UNII: 2968PHW8QP) Product Characteristics Color Score Shape Size Flavor BERRY (BERRY) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52268-012-01 2 in 1 CARTON 08/05/2010 1 177.4 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022372 08/05/2010 Labeler - Braintree Laboratories, Inc. (107904591) Establishment Name Address ID/FEI Business Operations BrainTree Laboratories, Inc. 617357954 MANUFACTURE(52268-012) , ANALYSIS(52268-012)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.