BIOMOX- amoxicillin tablet
Biomox by
Drug Labeling and Warnings
Biomox by is a Animal medication manufactured, distributed, or labeled by Virbac AH, Inc., Medispray Laboratories Private Ltd, Teva Pharmaceutical USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
NADA # 65-492, Approved by FDA
Biomox®
(amoxicillin tablets)
For use in DOGS only.DESCRIPTION:
BIOMOX® (amoxicillin tablets) are a broad-spectrum, semisynthetic antibiotic which provides bactericidal activity against a wide range of common gram-positive and gram-negative pathogens. Amoxicillin chemically is D-(-)α-amino-p-hydroxybenzyl penicillin trihydrate.Inactive Ingredients:
Dibasic Calcium Phosphate Dihydrate, Magnesium Stearate, Microcrystalline Cellulose and Sodium Starch Glycolate.ACTION:
Amoxicillin has bactericidal activity against susceptible organisms similar to that of ampicillin. It acts by inhibiting the biosynthesis of bacterial cell wall mucopeptides. Most strains of the following gram-positive and gram-negative bacteria have demonstrated susceptibility to amoxicillin both in vitro and in vivo: nonpenicillinase-producing staphylococci, alpha- and beta- hemolytic streptococci, Enterococcus faecalis, Escherichia coli and Proteus mirabilis. Amoxicillin does not resist destruction by penicillinase; therefore, it is not effective against penicillinase-producing bacteria, particularly resistant spahylococci. Most strains of Enterobacter and Klebsiella and all strains of Pseudomonas are resistant.
Amoxicillin may be given without regard to meals because it is stable in gastric acid. It is rapidly absorbed following oral administration and diffuses readily into most bodily fluids and tissues. It diffuses poorly into the brain and spinal fluid except when the meninges are inflamed. Most of the amoxicillin is excreted in the urine unchanged.INDICATIONS:
BIOMOX® (amoxicillin tablets) are indicated for the treatment of the following infections in dogs when caused by susceptible strains of organisms:BACTERIAL DERMATITIS due to Staphylococcus aureus, Streptococcus spp., Staphylococcus spp., and Escherichia coli.
SOFT TISSUE INFECTIONS (abscesses, wounds, lacerations) due to Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Proteus mirabilis, and Staphylococcus spp.
With all antibiotic therapy, appropriate in vitro cultures and sensitivities should be conducted prior to treatment.CONTRAINDICATIONS: Use of amoxicillin is contraindicated in animals with a history of allergic reaction to penicillin.
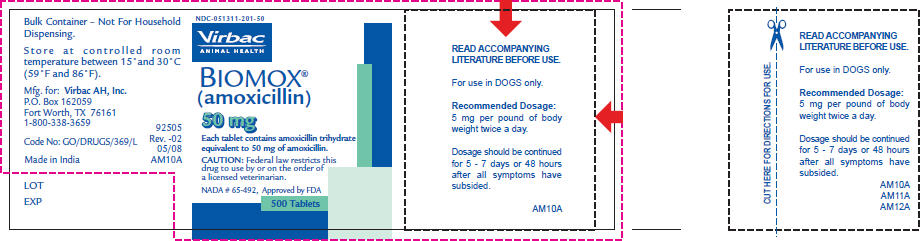
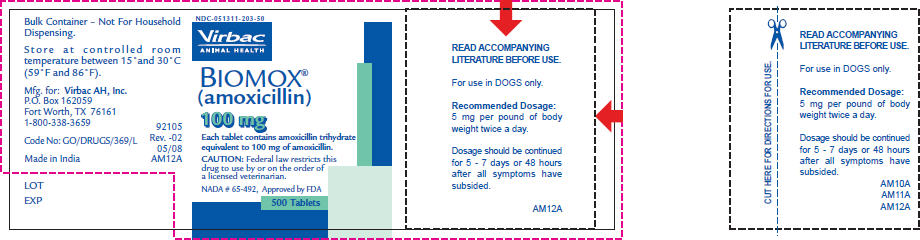
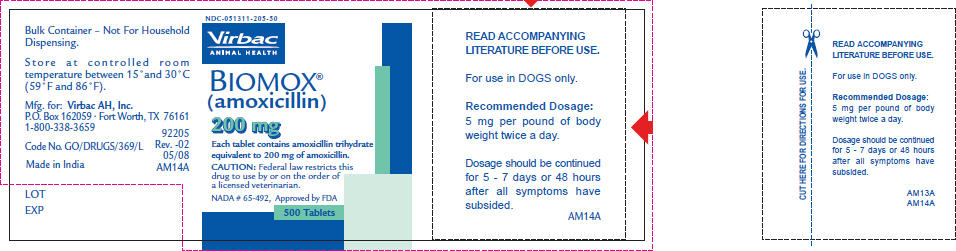
READ ACCOMPANYING LITERATURE BEFORE USE.
For use in DOGS only.
Recommended Dosage:
5 mg per pound of body weight twice a day.Dosage should be continued for 5 - 7 days or 48 hours after all symptoms have subsided.
ADVERSE REACTIONS:
Amoxicillin is a semisynthetic penicillin and, therefore, has the potential for producing allergic reactions. Epinephrine and/or steroids should be administered if an alleric reaction occurs.
WARNINGS:
For use in dogs only.
PRECAUTIONS:
Until adequate reproducitve studies are accomplished, BIOMOX® (amoxicillin tablets) should not be used in pregnant or breeding animals.
CAUTION:
Federal law restricts this drug to use by or on the order of a veterinarian.
DOSAGE AND ADMINISTRATION:
The recommended dosage is 5 mg per pound of body weight administered daily for 5 - 7 days or 48 hours after all symptoms have subsided. If no improvement is noted in 5 days, the diagnosis should be reconsidered and therapy changed.
SUPPLY:
BIOMOX® (amoxicillin tablets) are supplied in 50 mg, 100 mg and 200 mg concentrations in bottles of 500 tablets.
Manufactured for:
Virbac AH, Inc.
P.O. Box 162059
Fort Worth, TX 76161
1-800-338-3659Printed in the USA Rev. -05 04/18
©2018 Virbac Corporation
All Rights reserved
BIOMOX is a registered
trademark of Virbac AH, Inc. - INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
BIOMOX
amoxicillin tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 51311-201 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength amoxicillin (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 50 mg Product Characteristics Color WHITE Score no score Shape ROUND Size 6mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51311-201-50 500 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA065492 05/24/2010 BIOMOX
amoxicillin tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 51311-203 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength amoxicillin (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 100 mg Product Characteristics Color WHITE Score no score Shape ROUND Size 10mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51311-203-50 500 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA065492 05/24/2010 BIOMOX
amoxicillin tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 51311-205 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength amoxicillin (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 200 mg Product Characteristics Color WHITE Score no score Shape ROUND Size 11mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51311-205-50 500 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA065492 05/24/2010 Labeler - Virbac AH, Inc. (131568396) Establishment Name Address ID/FEI Business Operations Medispray Laboratories Private Ltd 915793457 MANUFACTURE Establishment Name Address ID/FEI Business Operations Teva Pharmaceutical USA, Inc. 611568031 api manufacture
Trademark Results [Biomox]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BIOMOX 74006481 1686993 Live/Registered |
BIOCRAFT LABORATORIES, INC. 1989-11-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.