NOREPINEPHRINE BITARTRATE injection, for intravenous use
Norepinephrine Bitartrate by
Drug Labeling and Warnings
Norepinephrine Bitartrate by is a Prescription medication manufactured, distributed, or labeled by Zydus Lifesciences Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NOREPINEPHRINE BITARTRATE- norepinephrine bitartrate injection, solution, concentrate
Zydus Lifesciences Limited
----------
NOREPINEPHRINE BITARTRATE injection, for intravenous use
Double click here to open Word Application.
Write ordered list in to word document.
Close Word Application.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 70771-1724-6
Norepinephrine Bitartrate Injection, USP
4 mg/4 mL (1 mg/mL)
FOR INTRAVENOUS INFUSION ONLY.
STERILE INJECTION
Warning: This is potent drug. Dosage should be controlled by frequent determination of blood pressure.
Do not leave patient unattended during administration. Avoid extravasation. Read package insert carefully.
DILUTE BEFORE USE. DISCARD UNUSED PORTION. PROTECT FROM LIGHT.
Warning: Contains Sulfites.
10 X 4 mL Single-Dose Vials
Rx only

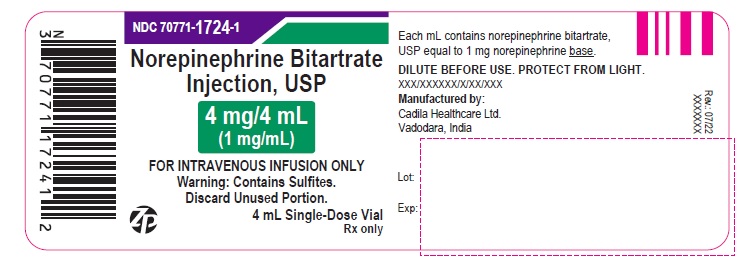
NDC: 70771-1724-1
Norepinephrine Bitartrate Injection, USP
4 mg/4 mL (1 mg/mL)
FOR INTRAVENOUS INFUSION ONLY
Warning: Contains Sulfites.
DISCARD UNUSED PORTION.
4 mL Single-Dose Vial
Rx only
| NOREPINEPHRINE BITARTRATE
norepinephrine bitartrate injection, solution, concentrate |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Zydus Lifesciences Limited (918596198) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zydus Lifesciences Limited | 873671928 | MANUFACTURE(70771-1724) , ANALYSIS(70771-1724) | |