ESOMEPRAZOLE MAGNESIUM DELAYED RELEASE- esomeprazole magnesium capsule, delayed release
Esomeprazole Magnesium Delayed Release by
Drug Labeling and Warnings
Esomeprazole Magnesium Delayed Release by is a Otc medication manufactured, distributed, or labeled by WALGREENS, TIME CAP LABORATORIES INC, MARKSANS PHARMA LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
DO NOT USE
Do not use if you have:
- trouble or pain swallowing food, vomiting with blood, or bloody or black stools
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
These may be signs of a serious condition. See your doctor.
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- adults 18 years of age and older
- this product is to be used once a day (every 24 hours), every day for 14 days
- may take 1 to 4 days for full effect
14-Day Course of Treatment
- swallow 1 capsule with a glass of water before eating in the morning
- take every day for 14 days
- do not take more than 1 capsule a day
- swallow whole. Do not crush or chew capsules
- do not use for more than 14 days unless directed by your doctor
Repeated 14-Day Courses (if needed)
- you may repeat a 14-day course every 4 months
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be caused by a serious condition.
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients
black iron oxide, eudragit, FD&C blue 1, FD&C red 3, ferric oxide yellow, gelatin, glyceryl monostearate, hydroxypropyl cellulose, hypromellose, magnesium stearate, polysorbate 80, potassium hydroxide, shellac, simethicone, sodium lauryl sulfate, sugar spheres, talc, titanium dioxide, triethyl citrate. - QUESTIONS
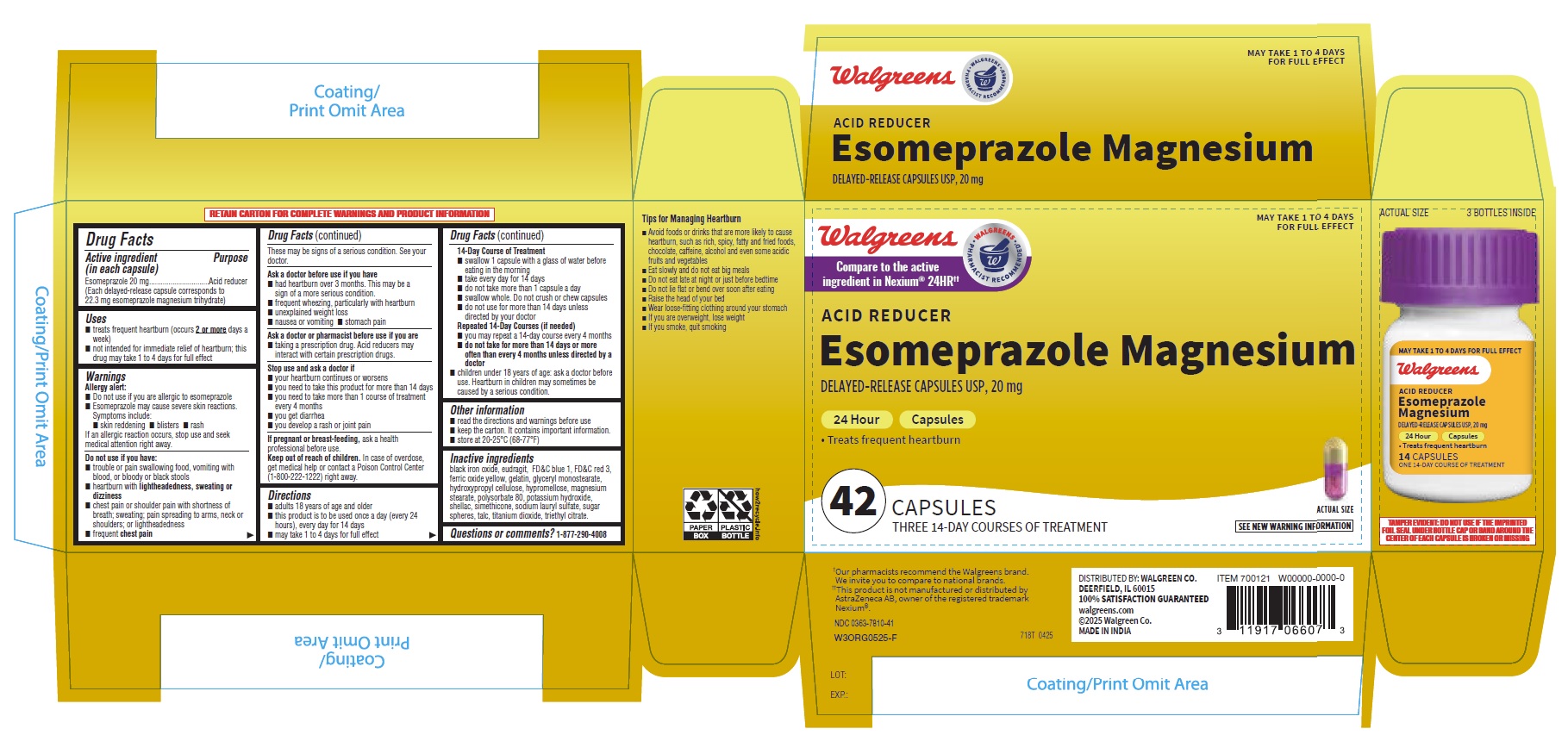
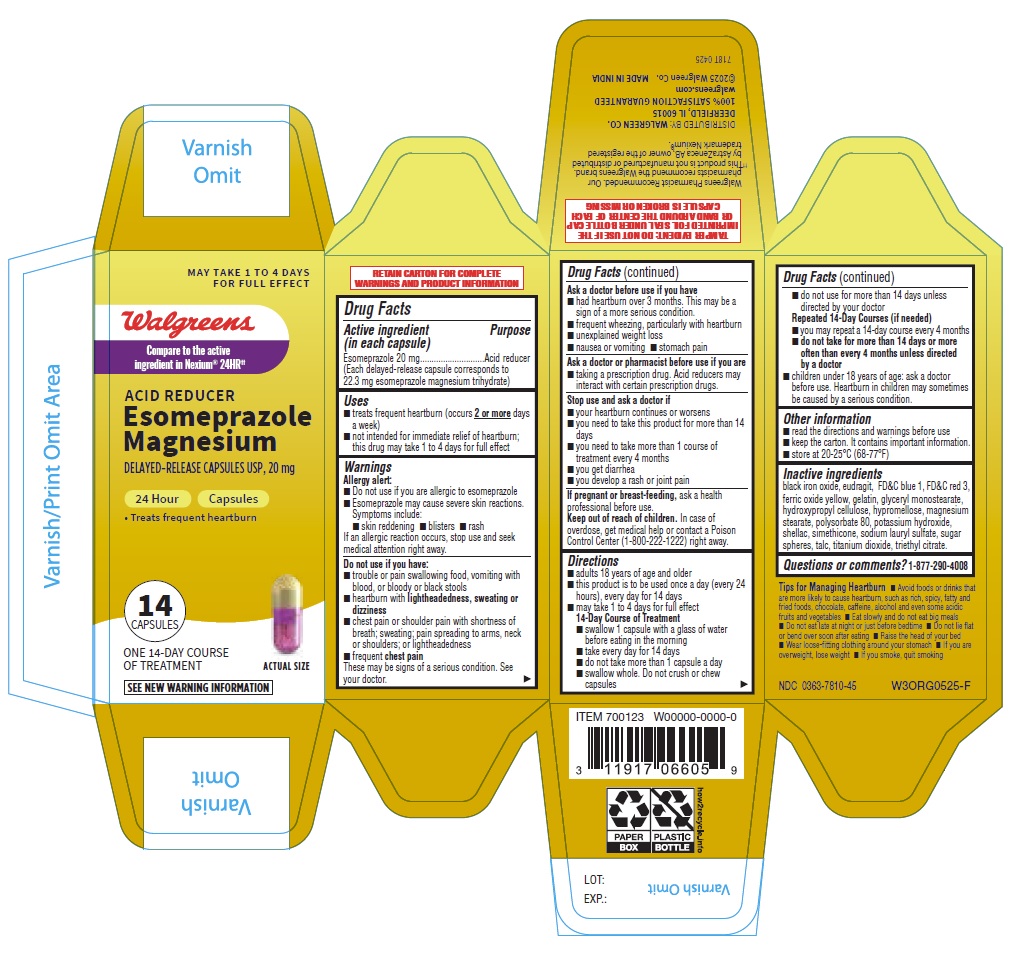
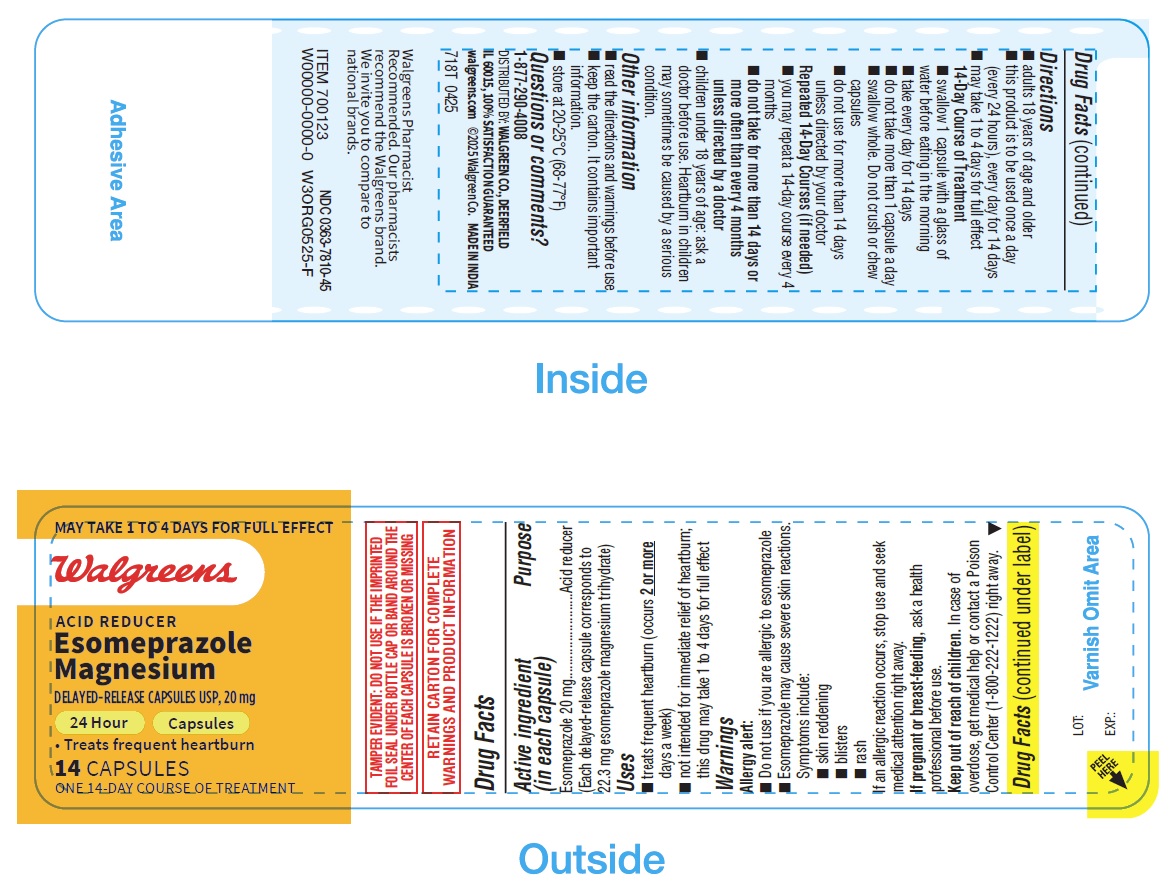
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ESOMEPRAZOLE MAGNESIUM DELAYED RELEASE
esomeprazole magnesium capsule, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0363-7810 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESOMEPRAZOLE MAGNESIUM (UNII: R6DXU4WAY9) (ESOMEPRAZOLE - UNII:N3PA6559FT) ESOMEPRAZOLE 20 mg Inactive Ingredients Ingredient Name Strength GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) SUCROSE (UNII: C151H8M554) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYPROMELLOSES (UNII: 3NXW29V3WO) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) DIMETHICONE (UNII: 92RU3N3Y1O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) GELATIN (UNII: 2G86QN327L) Product Characteristics Color purple ((golden cap and violet transparent body having light violet to violet colour band) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code 75 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0363-7810-45 1 in 1 CARTON 06/03/2025 1 14 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 0363-7810-82 2 in 1 CARTON 06/03/2025 2 14 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 0363-7810-41 3 in 1 CARTON 06/03/2025 3 14 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217264 06/03/2025 Labeler - WALGREENS (008965063) Registrant - TIME CAP LABORATORIES INC (037052099) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LIMITED 925822975 manufacture(0363-7810)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.