Nighttime Allergy Relief by MEIJER, INC. Drug Facts
Nighttime Allergy Relief by
Drug Labeling and Warnings
Nighttime Allergy Relief by is a Otc medication manufactured, distributed, or labeled by MEIJER, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NIGHTTIME ALLERGY RELIEF EXTRA STRENGTH- diphenhydramine hcl tablet
MEIJER, INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
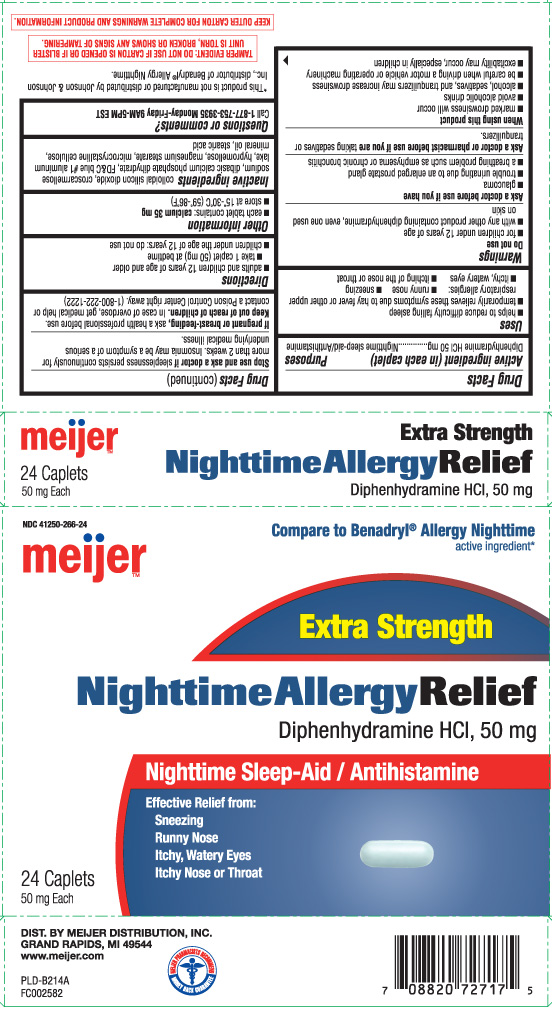
Drug Facts
Uses
- helps to reduce difficulty falling asleep
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Do not use
- for children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
When using this product
- marked drowsiness will occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Directions
- adults and children 12 years of age and older
- take 1 caplet (50 mg) at bedtime
- children under the age of 12 years: do not use
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, dibasic calcium phosphate dihydrate, FD&C blue #1 aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, mineral oil, stearic acid
Principal Display Panel
Compare to Benadryl® Allergy Nighttime active ingredient*
Extra Strength
Nighttime Allergy Relief
Diphenhydramine HCl, 50 mg
Nighttime Sleep-Aid / Antihistamine
Effective Relief from:
Sneezing
Runny Nose
Itchy, Watery Eyes
Itchy Nose or Throat
Caplets 50 mg each
DIST. BY MEIJER DISTRIBUTION, INC.
GRAND RAPIDS,MI 49544
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
| NIGHTTIME ALLERGY RELIEF
EXTRA STRENGTH
diphenhydramine hcl tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - MEIJER, INC. (006959555) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.