Fexofenadine Hydrochloride and Pseudoephedrine Hydrochloride Extended-Release Tablets USP
Fexofenadine Hydrochloride and Pseudoephedrine Hydrochloride by
Drug Labeling and Warnings
Fexofenadine Hydrochloride and Pseudoephedrine Hydrochloride by is a Otc medication manufactured, distributed, or labeled by McKESSON Corporation, Aurohealth LLC, Aurobindo Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FEXOFENADINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE - fexofenadine hydrochloride and pseudoephedrine hydrochloride tablet, film coated, extended release
Strategic Sourcing Services LLC
----------
Fexofenadine Hydrochloride and Pseudoephedrine Hydrochloride Extended-Release Tablets USP
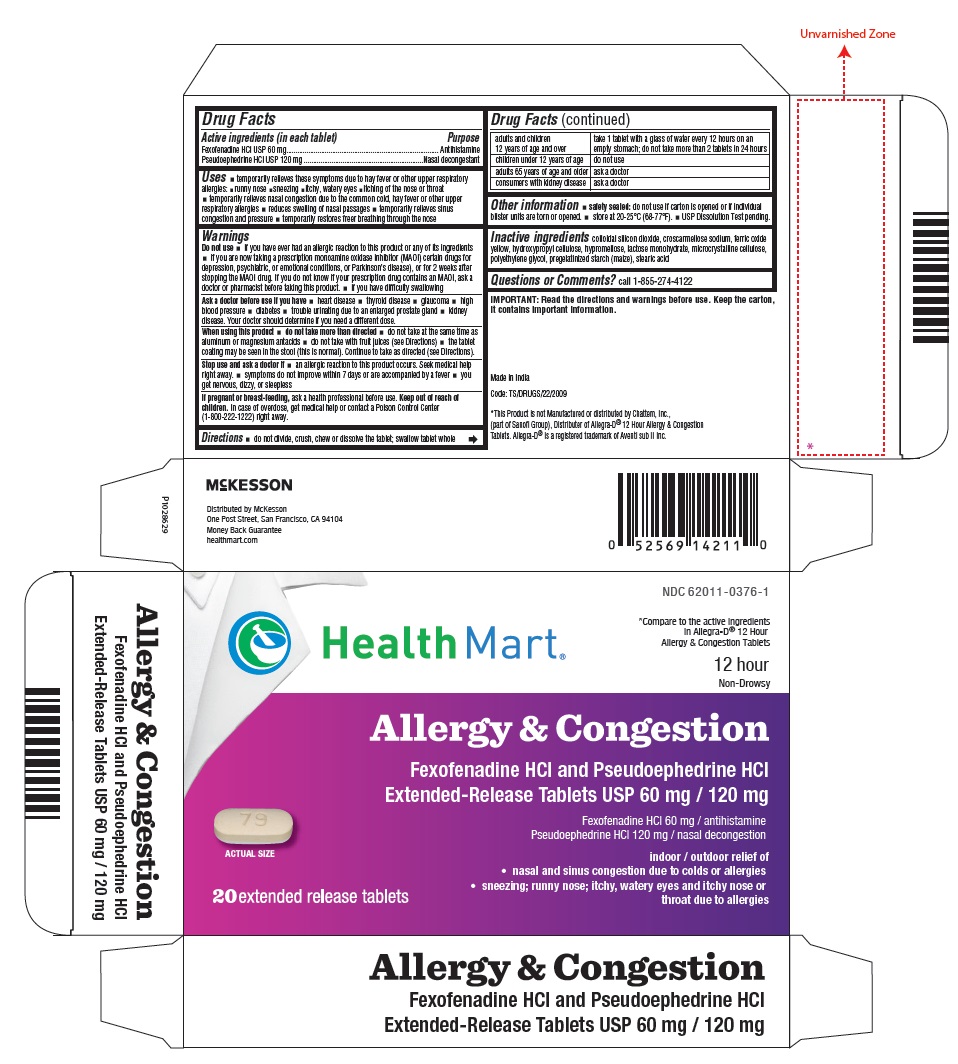
Drug Facts
Active ingredients (in each tablet)
Fexofenadine HCl USP 60 mg
Pseudoephedrine HCl USP 120 mg
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily restores freer breathing through the nose
Warnings
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have difficulty swallowing
Ask a doctor before use if you have
- heart disease
- thyroid disease
- glaucoma
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate gland
- kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- the tablet coating may be seen in the stool (this is normal). Continue to take as directed (see Directions).
Stop use and ask a doctor if
- an allergic reaction to this product occurs. Seek medical help right away.
- symptoms do not improve within 7 days or are accompanied by a fever
- you get nervous, dizzy, or sleepless
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- do not divide, crush, chew or dissolve the tablet; swallow tablet whole
| adults and children 12 years of age and over | take 1 tablet with a glass of water every 12 hours on an empty stomach; do not take more than 2 tablets in 24 hours |
| children under 12 years of age | do not use |
| adults 65 years of age and older | ask a doctor |
| consumers with kidney disease | ask a doctor |
Other information
- safety sealed: do not use if carton is opened or if individual blister units are torn or opened.
- store at 20-25°C (68-77°F).
- USP Dissolution Test Pending.
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, ferric oxide yellow, hydroxypropyl cellulose, hypromellose, lactose monohydrate, microcrystalline cellulose, polyethylene glycol, pregelatinized starch (maize), stearic acid
Questions or Comments?
call 1-855-274-4122
IMPORTANT: Read the directions and warnings before use. Keep the carton, it contains important information.
Distributed by McKesson
One Post Street, San Francisco, CA 94104
Money Back Guarantee
healthmart.com
Made in India
Code: TS/DRUGS/22/2009
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 2 x 10 Blister Carton
NDC: 62011-0376-1
Compare to the active ingredients
in Allegra-D® 12 Hour
Allergy & Congestion Tablets
12 hour
Non-Drowsy
Health Mart®
Allergy & Congestion
Fexofenadine HCl and Pseudoephedrine HCl
Extended-Release Tablets USP 60 mg / 120 mg
Fexofenadine HCl 60 mg / antihistamine
Pseudoephedrine HCl 120 mg / nasal decongestion
indoor / outdoor relief of
- nasal and sinus congestion due to colds or allergies
- sneezing; runny nose; itchy, watery eyes and itchy nose or throat due to allergies
20 extended release tablets
| FEXOFENADINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE
fexofenadine hydrochloride and pseudoephedrine hydrochloride tablet, film coated, extended release |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Strategic Sourcing Services LLC (116956644) |
| Registrant - Aurohealth LLC (078728447) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aurobindo Pharma Limited | 650381903 | ANALYSIS(62011-0376) , MANUFACTURE(62011-0376) | |