V-GO DISPOSABLE INSULIN DELIVERY DEVICE-
V-GO DISPOSABLE INSULIN DELIVERY DEVICE by
Drug Labeling and Warnings
V-GO DISPOSABLE INSULIN DELIVERY DEVICE by is a Other medication manufactured, distributed, or labeled by Valeritas, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Using the V-Go™ Disposable Insulin Delivery DeviceInstructions for Patient Use
Read the entire manual before operating the V-Go™ Disposable Insulin Delivery Device.
CONTENTS
Section 1 (Part 1): Introduction and V-Go Overview
Healthcare professional prescribing considerations
Valeritas customer care
Liability disclaimerSection 1 (Part 2): Before You Begin
Section 2 (Part 1): V-Go Operating Instructions
V-Go product overview
EZ Fill product overview
Aseptic techniqueSection 2 (Part 2): Filling the V-Go with Insulin Using the EZ Fill
Step 1: Remove the plug from the EZ Fill drawer
Step 2: Place the V-Go into the EZ Fill
Step 3: Place the insulin vial into the EZ Fill insulin vial holder
Step 4: Fill the V-Go with insulin
Step 5: Remove the V-Go from the EZ Fill
Step 6: Store the EZ Fill and unfilled V-Go devices after each day's V-Go filling session
Step 7: Remove an empty vial of insulin from the insulin vial holder and place a new vial of insulin into the insulin vial holder
Step 8: Dispose of the EZ FillSection 2 (Part 3): Applying and Using the V-Go
Step 1: Inspect the V-Go before you attach it to your body
Step 2: Wash your hands
Step 3: Select and prepare the infusion site
Step 4: Attach the V-Go
Step 5: Start the 24-hour flow of insulin with the V-Go
Step 6: Bolus dose with the V-Go at mealtimes
Step 7: Monitor V-Go progress
Step 8: Remove the V-Go
Step 9: Dispose of the V-GoSection 3: V-Go Safety Information
Caution
Indication
Healthcare professional dosing considerations
Warnings
Precautions
Adverse reactionsSection 4: Product Specifications
EZ Fill
V-Go
Delivery accuracy test resultsSection 5: V-Go User Assistance Information
Section 6: Troubleshooting/Frequently Asked Questions
Section 7: Glossary and Packaging Symbols
The V-Go Disposable Insulin Delivery Device should be used only by people who have been prescribed the device, and only for intended use.
Terms in bold and italic are explained in the glossary.
SECTION 1 (Part 1): INTRODUCTION AND V-Go™ OVERVIEW
The V-Go provides a continuous subcutaneous insulin infusion over 24 hours. It uses a preset basal rate for between-meal and nighttime insulin. The V-Go provides on-demand bolus dosing to cover glucose intake at meals. The V-Go device is convenient and easy to operate (see Section 3 for indication).
Here is how to use the V-Go:
- Fill the V-Go with insulin using the EZ Fill
- Attach the V-Go to a selected site (skin area)
- Push in the needle button to deliver the preset basal rate
- Wear the V-Go for one (1) 24-hour period
- Bolus dose at meals as prescribed by your doctor or healthcare professional
- At the end of the 24-hour period, retract the needle and remove the V-Go from your body
- Discard the device after use (the V-Go is 100% disposable)
- Repeat these steps for the next 24-hour period using a new V-Go
Healthcare Professional Prescribing Considerations
Dosing considerations
- When selecting a V-Go option, healthcare professionals should refer to their own experience when initiating continuous subcutaneous insulin infusion therapy with a patient. If unfamiliar, the healthcare professional should refer to insulin therapy guidelines from diabetes associations.
- The following should be considered when initially prescribing the V-Go:
- - Understand the total daily dose of insulin your patient is actually taking with their current insulin regimen versus what is being prescribed. Selecting the correct V-Go option may lessen the risk of hypoglycemia (low blood sugar).
- - It is common practice to reduce the total daily insulin dose when starting a patient on continuous subcutaneous insulin infusion therapy and this reduction should be considered when starting a patient on the V-Go.
Other prescribing considerations
- A separate prescription of a U100 fast-acting insulin is required for use with the V-Go (see Section 3 for insulins tested with the V-Go).
- - Two (2) vials of insulin are required for the V-Go 20 option.
- - Three (3) vials of insulin are required for the V-Go 30 and 40 options.
The V-Go comes in 3 options for your insulin needs. Your doctor or healthcare professional has selected the most appropriate V-Go option for you.
The 3 V-Go options are:

- – 20 Units/24 hr (0.83 U/hr) basal rate and up to 36 Units of on-demand bolus dosing in 2-Unit increments1

- – 30 Units/24 hr (1.25 U/hr) basal rate and up to 36 Units of on-demand bolus dosing in 2-Unit increments1

- – 40 Units/24 hr (1.67 U/hr) basal rate and up to 36 Units of on-demand bolus dosing in 2-Unit increments1
- 1 36 Units of insulin are available for on-demand bolus dosing in all V-Go options. Bolus doses are delivered in 2-Unit increments. You can only push the bolus delivery button 18 times in every 24-hour period. Each push of the bolus delivery button delivers 2 Units of insulin (1 push = 2 Units).
IMPORTANT: A separate prescription for insulin is required for use with the V-Go. A U100 fast-acting insulin should be used to fill the V-Go. (See Section 3 for insulins tested with the V-Go.) - V-Go 20 requires two (2) vials of insulin
- V-Go 30 and 40 require three (3) vials of insulin
IMPORTANT: Let your healthcare professional know how much insulin you actually take each day. Your healthcare professional will help select the correct V-Go option for you. The correct V-Go option may lessen the risk of hypoglycemia. SECTION 1 (Part 2): BEFORE YOU BEGIN
Step 1: Remove the EZ Fill from the blister packaging

- A.
Turn the packaging over so you can read the text.
- B. Peel back on the lower right corner.
- C. Remove the EZ Fill.
- B. Peel back on the lower right corner.
Step 2: Remove the V-Go from the blister packaging

- A.
Turn the packaging over so you can read the text.
- B. Peel back on the lower left corner on one of the V-Go containers.
- C. Remove the V-Go. Do not pull on the adhesive tab while removing the V-Go from the container.
- B. Peel back on the lower left corner on one of the V-Go containers.
SECTION 2 (Part 1): V-Go OPERATING INSTRUCTIONS
IMPORTANT: Understanding the button names and locations is important for the successful use of the V-Go and EZ Fill. V-Go Product Overview
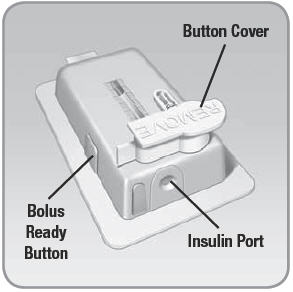
- Button Cover: Covers the needle button. Prevents the needle button from being pushed in. Cover must be removed for V-Go to operate.
- Insulin Port: Location where insulin enters the V-Go through the EZ Fill.
- Bolus Ready Button: A grey button that lies flat on one of the long edges of the V-Go. When activated, it releases the bolus delivery button. This allows for bolus dosing of insulin.
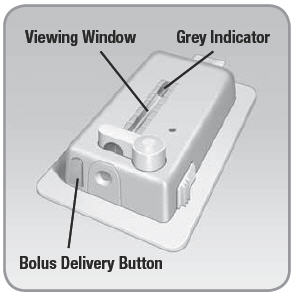
- Bolus Delivery Button: A grey button next to the insulin port on one of the short edges of the V-Go. When pressed after the bolus ready button activates this button, the V-Go delivers a bolus dose of 2 Units of insulin (1 push = 2 Units).
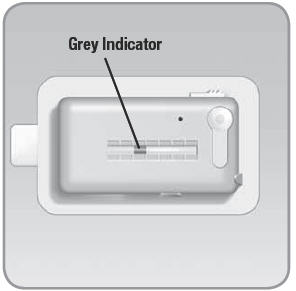
- Viewing Window: Shows a view of the insulin reservoir. A grey indicator in the window demonstrates that insulin is flowing from the device.
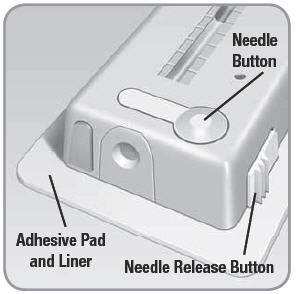
- Needle Button: When pressed, it inserts the needle into your skin and begins the flow of insulin into your body.
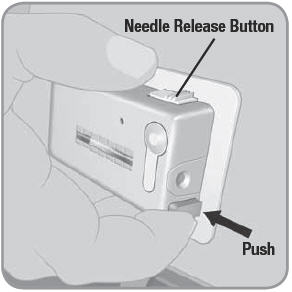
- Needle Release Button: A wide white button with 3 ridges on one of the long edges of the V-Go. An activated needle release button removes the needle from your body and stops the V-Go from delivering insulin.
- Adhesive Pad and Liner: Once the protective adhesive liner is removed, the adhesive pad affixes the V-Go to your skin.
EZ Fill Product Overview
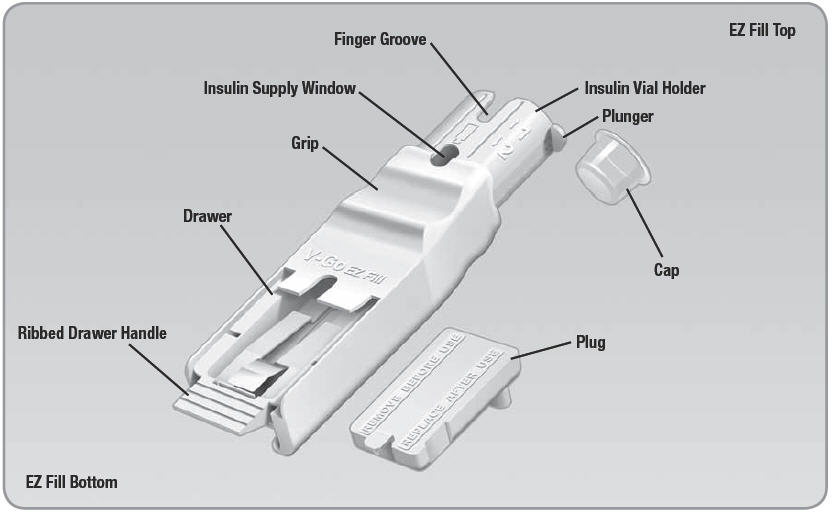
- Cap: Protects the needle inside the EZ Fill (bottom of insulin vial holder).
- Finger Groove: Opening to allow for easy removal of the insulin vial.
- Insulin Vial Holder: Opening in the EZ Fill where the insulin vial is placed upside down.
- Insulin Supply Window: Shows a view of the insulin remaining in the insulin vial.
- Plunger: When you pull this handle slowly up (about 5 seconds) and then push it down (about 25 seconds), insulin transfers from the vial into the V-Go™.
- Grip: The left thumb occupies this groove throughout most of the filling process.
- Drawer: The V-Go is placed in this drawer for filling. The plug sits in the drawer when the EZ Fill is not in use.
- Ribbed Drawer Handle: A grooved piece at the bottom end of the EZ Fill that helps with opening and closing the drawer.
- Plug: When placed in the drawer, the plug protects the EZ Fill components between each fill.
Aseptic Technique
Aseptic technique helps keep the V-Go and the EZ Fill devices clean during preparation, filling, and application. Following this technique requires hand-washing and wiping the application site with an alcohol swab. It may involve wiping the V-Go device with an alcohol swab if the device comes into contact with anything else.
Use aseptic technique when you are preparing, filling, and attaching a new V-Go.
- 1.
Inserting a needle into your skin creates an opening where germs can enter your body. This could cause an infection at the infusion site. Always use aseptic technique. The V-Go, EZ Fill, and other supplies come to you sterilized. To keep them that way, follow these precautions:
- a.
Always wash your hands thoroughly before preparing, filling, and attaching the V-Go.
- b. The infusion needle is sterile and protected within the V-Go. Do not touch the underside of the V-Go after you remove it from the sterile blister packaging. If you place the V-Go on a surface, do not let the underside touch that surface. Gently lay the V-Go on the front side of the device.
- 2. If the underside of the V-Go touches something, you must wipe the underside with an alcohol swab.
- 3. Do not touch the circular opening on the top of the EZ Fill plug. If you place the plug on a surface, do not let the circular opening touch that surface. If the circular opening touches something, you must wipe the circular opening with an alcohol swab.
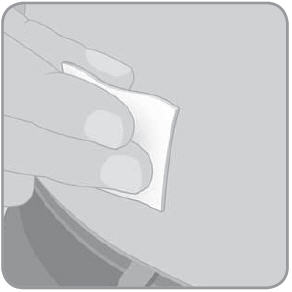
- 4. Wipe the infusion site of your skin with an alcohol swab. Let the alcohol dry before you attach the V-Go. Do not touch this site again before putting the V-Go on your skin.
- a.
Always wash your hands thoroughly before preparing, filling, and attaching the V-Go.
SECTION 2 (Part 2): FILLING THE V-Go™ WITH INSULIN USING THE EZ FILL
IMPORTANT: The EZ Fill is intended to fill one (1) V-Go each day. Use the EZ Fill to fill 30 V-Go devices, one (1) V-Go each day. Then discard the EZ Fill after 30 fills. A new EZ Fill will be included in each month’s supply of V-Go devices. Do not fill and store V-Go devices ahead of time. That may result in the loss of insulin effectiveness. Remove the EZ Fill from the refrigerator, allowing the insulin to reach room temperature (about 20 minutes) before filling the V-Go. Step 1: Remove the plug from the EZ Fill drawer
NOTE: On day 1 when you remove the EZ Fill from the packaging, the plug will be separate from the EZ Fill; therefore, you can skip Step 1. 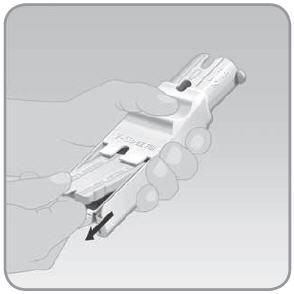
- A.
Place your thumb over the plug tab and your
forefinger underneath the ribbed drawer handle. Pull the drawer out until it stops. The plug will pop up from the drawer.
- B. Lift the plug out of the EZ Fill using your thumb and forefinger. Set the plug aside. You will replace the plug into the EZ Fill after you fill the V-Go with insulin.
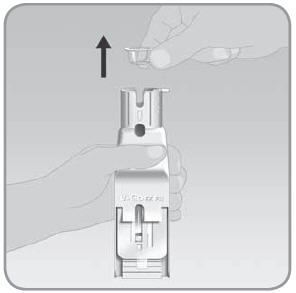
Step 2: Place the V-Go into the EZ Fill
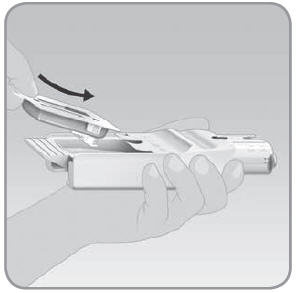
- A.
Hold the EZ Fill so that the plunger is on the top right side of the EZ Fill.
- B. With your other hand, place the V-Go into the EZ Fill drawer upside down. Place the adhesive side facing up with the plastic tab toward the bottom of the EZ Fill. The button cover should be facing down into the drawer and toward the top of the EZ Fill.
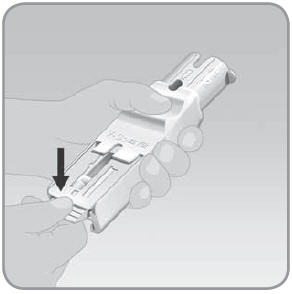
- C.
Slide the V-Go up into the EZ Fill drawer. Press
the V-Go flat with the top of the drawer. Hold the V-Go down with your thumb.
- Make sure that the adhesive backing on the V-Go fits inside the drawer.
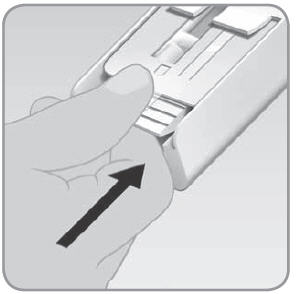
- D. Push the drawer all the way closed.
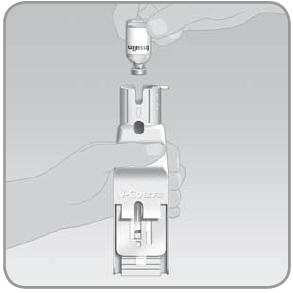
Step 3: Place the insulin vial into the EZ Fill insulin vial holder
NOTE: Before placing a new vial into the EZ Fill, check to make sure the insulin vial is not expired and that it will not expire during the time period that the vial will be used in the EZ Fill. 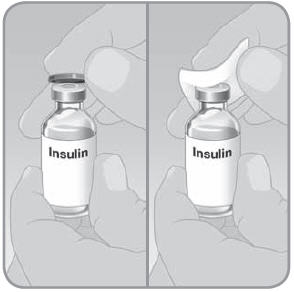
- A. Remove the protective cap from the insulin vial. Wipe the top with an alcohol swab.
- B. Pull the EZ Fill cap out of the insulin vial holder and discard.
- C.
Turn the insulin vial upside down so the top of the vial faces downward.
- D. Place the insulin vial into the EZ Fill. Push the vial firmly straight down into the insulin vial holder until you feel it attach and you cannot push it further.
- E. Check the insulin supply window to make sure there is enough insulin to fill the V-Go™.
- D. Place the insulin vial into the EZ Fill. Push the vial firmly straight down into the insulin vial holder until you feel it attach and you cannot push it further.
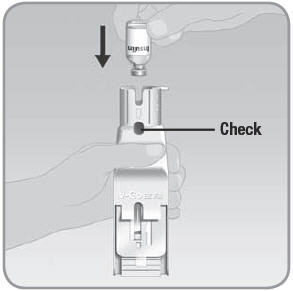
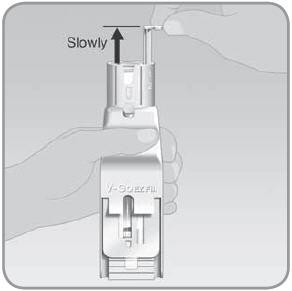
Step 4: Fill the V-Go with insulin
- A.
Hold the EZ Fill upright over a counter or table by placing your thumb across the grip. The grip is just below the insulin vial holder and the plunger. Use the rest of your hand to support the back of the EZ Fill.
- Do not block light from the back of the EZ Fill. Light from the back will help you see the viewing window.
NOTE: Keep the EZ Fill in a vertical, fully upright position during filling. Keep the viewing window at eye level. Direct the backside of the EZ Fill toward a brightly lit area when you fill the V-Go. This makes it easier to see the flow of insulin. Seeing the flow of insulin helps you fill the V-Go properly and completely. 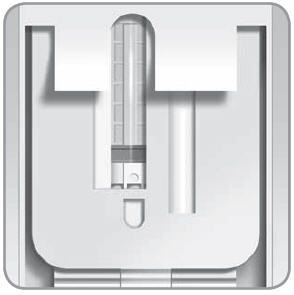
- C. Make sure the viewing window is at eye level so you can watch the V-Go fill with insulin.
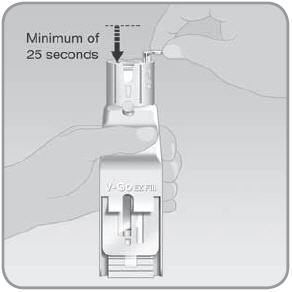
- D.
Slowly and steadily push the plunger down over a minimum of 25 seconds to fill the V-Go™ with insulin.
- The V-Go should be filled with insulin in a continuous flow of droplets, not in a solid, steady stream. Make sure you don't fill the V-Go with a steady stream of insulin. If you push too fast or too hard the insulin will become foamy. Foamy insulin may lead to an incomplete fill. If the insulin turns foamy, push the plunger more slowly.
- E.
Push the plunger until it is all the way down.
- When the position of the plunger is all the way down, the V-Go should be full.
IMPORTANT: Make sure the V-Go fills with insulin in a continuous flow of droplets, not in a solid, steady stream. Make sure the insulin is not foamy. Foam will not damage the V-Go or the insulin, but may lead to an incomplete fill of the V-Go. Count to about 25 seconds when you push the plunger down to make sure the V-Go is filled with insulin. - F.
Check that the V-Go is filled properly. Ideally you should see only fluid and no air space. You may see tiny air bubbles smaller than a grain of rice.
- If the V-Go is not filled fully, check to see if you still have insulin in your vial.
- – If you do not, replace vial (Step 3) and repeat Step 4A to 4F.
- – If you do, repeat Step 4A to 4F.
- If the V-Go is not filled fully, check to see if you still have insulin in your vial.
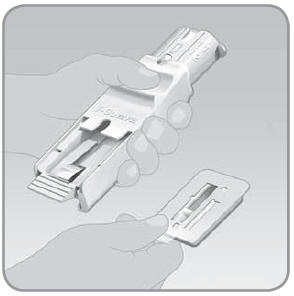
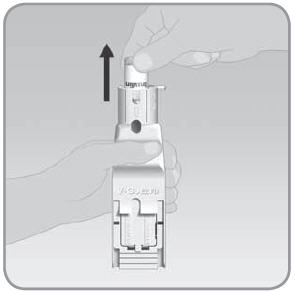
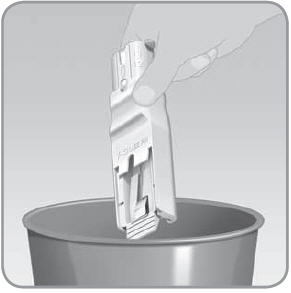
Step 5: Remove the V-Go from the EZ Fill

- A.
Hold the EZ Fill in a sideways position.
- B. Pull the EZ Fill ribbed drawer handle all the way out. Pull until the V-Go pops up from the drawer.
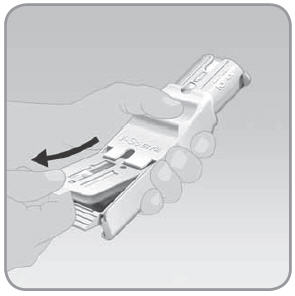
- C.
Remove the filled V-Go from the EZ Fill by lifting it up with your thumb and forefinger.
- Leave the plunger in the down position.
Step 6: Store the EZ Fill and unfilled V-Go™ devices after each day's V-Go filling session
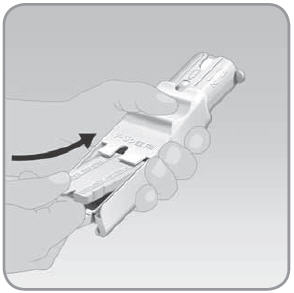
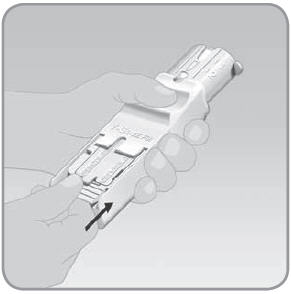
- A. Place the plug back into the EZ Fill. Follow the directions for filling Step 2A to 2D. This time use the plug and not a V-Go.
NOTE: Do not remove the insulin vial from the EZ Fill. The insulin vial should be removed only when the vial is empty. Additional Steps
Step 7: Remove an empty vial of insulin from the insulin vial holder and place a new vial of insulin into the insulin vial holder
SECTION 2 (Part 3): APPLYING AND USING THE V-Go™
Step 1: Inspect the V-Go before you attach it to your body
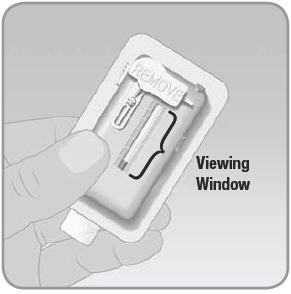
- A.
Look at the entire viewing window to make sure that the V-Go has been filled completely with insulin. You should see only fluid and no air space.
- B. Rotate the V-Go and look in the viewing window. The viewing window will show you if there are any large air bubbles.
IMPORTANT: If you see air bubbles larger than a grain of rice, the V-Go may not be filled completely. Repeat Step 4A to 4F in Section 2 (Part 2) to ensure a complete fill. Having a few small bubbles is normal and harmless. As discussed in Section 2 (Part 2), you should slowly and steadily push the plunger down for about 25 seconds to reduce air bubbles as you fill the V-Go. Step 2: Wash your hands
Always wash your hands thoroughly before preparing the V-Go for use. Use aseptic technique when you are preparing and applying a new V-Go. See the end of Section 2 (Part 1) for a description of aseptic technique.
Step 3: Select and prepare the infusion site
IMPORTANT: The place on your body where you attach the V-Go is important for the success of your therapy. Choose a location that remains flat when you are sitting down, standing up, or lying down. Discuss the best location for you with your doctor or healthcare professional. 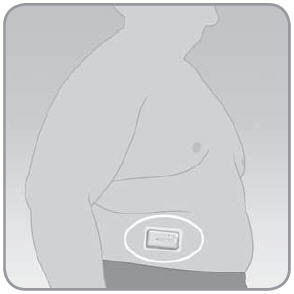
- A.
The V-Go can be worn any place that insulin can be injected or infused. Insulin is injected or infused into the subcutaneous tissue.
- You may wish to attach the V-Go:
- –
On the abdomen. The abdomen has ample flat surface area, and is an accessible and comfortable location. Insulin absorption is fast, predictable, and less affected by exercise when administered through the abdomen.
- If the V-Go is worn on the abdomen, keep it horizontal above the belt line.
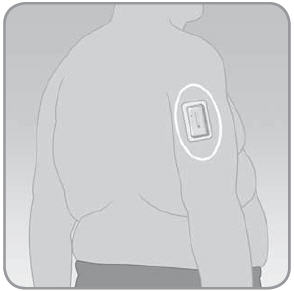
- –
On the backside of the arm, not on the muscle.
- If the V-Go is worn on the backside of the arm, keep it in the up and down direction. Make sure you can see the grey indicator in the viewing window directly by rotating your arm, or indirectly through the use of a mirror. Attach the V-Go in the up and down position as your arm hangs down.
- You may wish to attach the V-Go:
- B. When choosing the location for the V-Go™, consider the following:
- Make sure you are able to view the grey indicator in the viewing window, either directly or indirectly through the use of a mirror.
- That you can comfortably reach the V-Go and all of the buttons, for easy operation and removal.
- That you apply the V-Go to a flat area of skin, not on a fold of the skin, muscle, or bone.
- That the site is flat when you are in any of the following positions: sitting down, standing up, or lying down.
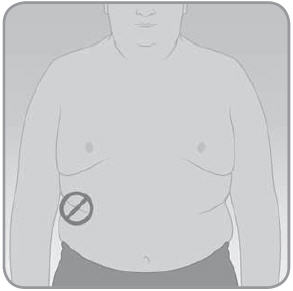
- C.
When choosing the location for the V-Go, avoid the following:
- The belt line or waistline, or other areas where clothing may rub or constrict.
- Areas with excessive hair. You may shave the area to help the V-Go attach to your skin.
- Areas that are curved or rigid due to muscle or bone.
- Areas within a 1-inch circle around the belly button or surgical scars.
- Skin that is tender, bruised, red, or hard or has any skin disease or infection.
NOTE: If you have sensitive skin or your skin becomes irritated, ask your doctor or healthcare professional about skin barrier products. Step 4: Attach the V-Go
Actions in this step describe how to attach the V-Go to your abdomen. As noted in Section 2 (Part 3) Step 3, there are a couple of places you can attach the V-Go.
- A.
Practice placing the V-Go before you actually remove the protective liner. Practice helps you to make sure you can reach and operate the bolus ready button, bolus delivery button, needle button, and needle release button.
- To practice with your right hand, hold your thumb on the needle release button, your index finger near but not on the bolus delivery button, and middle finger near the bolus ready button.
- Hold the V-Go on the selected body site to make sure that you can reach all necessary buttons when you wear the V-Go.
Now you are ready to attach the V-Go.
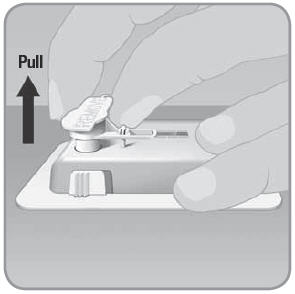
- B.
Remove the button cover.
- 1. Hold the V-Go™ with one hand and pull the button cover with the other hand. Pull the button cover in a quick, straight direction up and away from the V-Go.
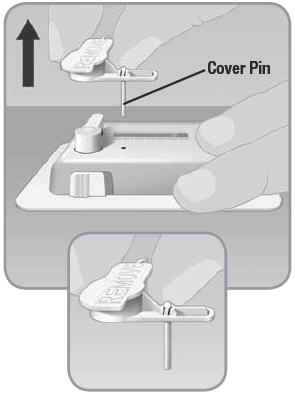
- 2.
Check to see if the cover pin came off with the button cover.
- If the cover pin is not attached and has remained inside the V-Go, try to remove the pin manually; otherwise discard this V-Go and start over with a new V-Go.
IMPORTANT: If you bend the button cover backwards, as you would a soda pop can top, you could damage the V-Go. 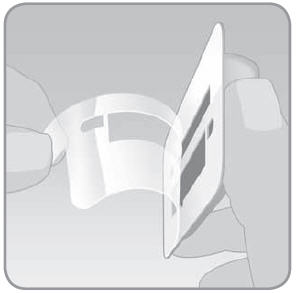
- C.
Lift the adhesive tab and completely peel off the adhesive liner from the bottom of the V-Go. Removing the liner exposes the adhesive.
- 1.
Do not touch the adhesive surface. Touching the adhesive surface can reduce the strength of the adhesive.
- 2. Keep the adhesive pad intact and clean before you place it on the infusion site.
- 1.
Do not touch the adhesive surface. Touching the adhesive surface can reduce the strength of the adhesive.
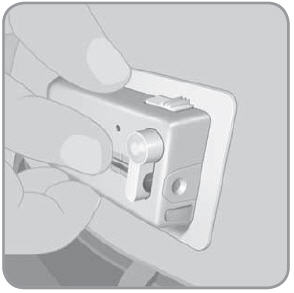
- D.
Without touching the adhesive pad, press the V-Go against the skin at the cleaned needle infusion site.
- E. Hold the V-Go in place for 5-10 seconds. Run your finger around the entire edge of the adhesive pad to make sure it is attached to your body. Do not accidentally press any of the buttons.
Step 5: Start the 24-hour flow of insulin with the V-Go
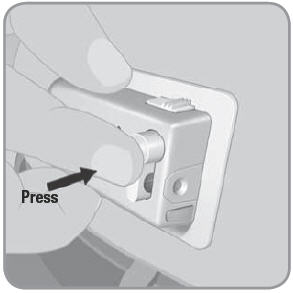
Press down on the raised bump of the needle button with one firm quick motion. Needle button needs to be pressed completely into the V-Go until you hear a click or button locks in place. This begins the flow of insulin. The V-Go delivers a continuous preset basal rate of insulin over 24 hours.
NOTE: Grasp the side of the V-Go as you press the raised bump of the needle button to keep the V-Go in place. Be careful not to press any other buttons during this process. IMPORTANT: Avoid exposing the V-Go™ to direct sunlight. Avoid extremely hot temperatures. Remove the V-Go prior to hot tub, whirlpool, or sauna use and replace with a new filled V-Go afterward. IMPORTANT: If the needle button does not fully depress or does not remain in the down position, the V-Go cannot function. You will not receive the preset basal rate of insulin. Do not use the V-Go if the needle button does not stay down. Remove and discard that V-Go. Start over with a new V-Go. Once the needle button is pushed down and locks into place, insulin is now flowing into your body. The needle within the V-Go remains in your body for the entire 24-hour use of the V-Go. The V-Go design helps to minimize discomfort. Step 6: Bolus dose with the V-Go at mealtimes
Your doctor or healthcare professional should have instructed you on how and when to use the V-Go and how many bolus doses to administer at mealtimes. Consult your doctor or healthcare professional if you have questions regarding your specific bolus dosing needs.
IMPORTANT: Each V-Go has a total of 36 Units of insulin available for on-demand bolus dosing per 24-hour period. The 36 Units of insulin can only be delivered in 2-Unit increments (1 push = 2 Units). You can only push the bolus delivery button 18 times in every 24-hour period. After 18 uses, the bolus delivery button will pop out and lock, preventing further bolus doses. Plan your day so that you have enough insulin for each meal. 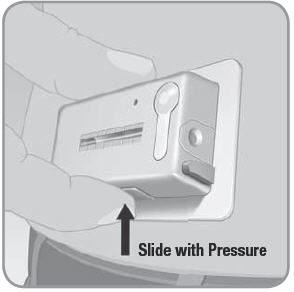
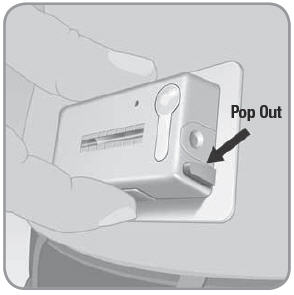
- A.
To administer 2 Units of insulin at mealtime:
- 1.
Slide your finger with pressure to locate and press the bolus ready button on the long edge of the V-Go.
- The bolus delivery button located at the end of the V-Go will pop out. You will hear a click.
- 1.
Slide your finger with pressure to locate and press the bolus ready button on the long edge of the V-Go.
- 2.
Push the bolus delivery button all the way into the V-Go until it stops. You will hear another click.
- You have just delivered 2 Units of insulin (1 push = 2 Units).
IMPORTANT: Do not touch the needle release button (button with ridges) while giving yourself bolus doses. This may cause you to retract the needle before the end of its 24-hour use and stop the delivery of insulin to your body. If this occurs and the needle button pops out, remove the V-Go and replace it with a new one. - B. Repeat Step 6A until you reach the number of bolus doses prescribed by your doctor or healthcare professional for that specified time.
IMPORTANT: If you do not know the number of insulin units you have just delivered or if you lose count, STOP bolus dosing, monitor your blood glucose, and follow the plan agreed upon with your doctor or healthcare professional. If you have not agreed upon a plan already, consult your doctor or healthcare professional for guidance. Step 7: Monitor V-Go progress
- A.
While wearing the V-Go, you should check your blood glucose at regular intervals to make sure the V-Go is working properly. Refer to Section 3 for recommendations on blood glucose monitoring.
- B. You can also check the movement of the grey indicator in the viewing window to make sure the V-Go is working properly. After several hours, you should see a slight change in the location of the grey indicator as it moves toward the needle button.
- The exact location of the grey indicator will vary based on the number of bolus doses you have used.
- B. You can also check the movement of the grey indicator in the viewing window to make sure the V-Go is working properly. After several hours, you should see a slight change in the location of the grey indicator as it moves toward the needle button.
IMPORTANT: The indicator moves very slowly over the 24-hour period. It will take time to notice the change in the indicator location. Monitoring the V-Go progress is important to make sure the V-Go is delivering insulin. If the indicator does not appear to be moving after several hours of wearing the V-Go, consider checking your blood glucose levels. If your levels are abnormally high after normal V-Go use as prescribed by your doctor or healthcare professional, you should remove the V-Go and replace it with a new one. Step 8: Remove the V-Go
IMPORTANT: After 24 hours of use it is time to replace the current V-Go with a new filled V-Go. Remember to fill the V-Go with insulin using the EZ Fill, and attach the V-Go to your skin immediately after filling. The V-Go is meant to be worn for 24 hours only. Establishing a daily routine will help remind you to change the V-Go every 24 hours. - A.
Start by filling a new V-Go with insulin. Follow previous instructions in Section 2 (Part 2) Steps 1 to 6.
- Make sure the V-Go is completely filled with insulin before you attach.
- B. Set the new filled V-Go aside until you remove the used V-Go from your skin.
NOTE: Do not block the needle button with your finger or any part of your hand or clothing while releasing the needle via the needle release button. 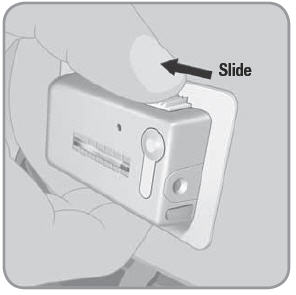
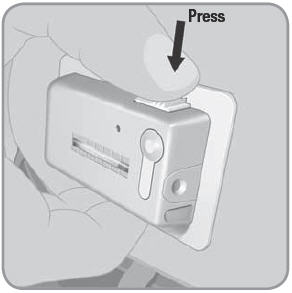
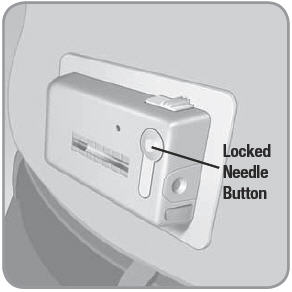
- C. To remove the used V-Go™, place thumb on the ridged section of the needle release button. Slide the needle release button along the side of the V-Go. In a continuous motion, press the needle release button directly into the V-Go until you hear a click. This motion retracts the needle.
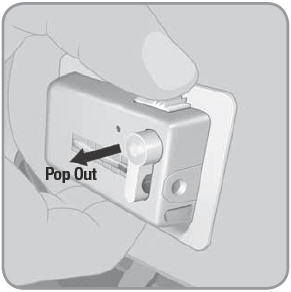
- D. At this point the needle button will pop out automatically.
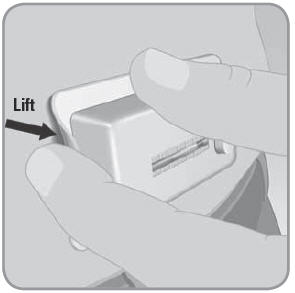
- E. To remove the V-Go from your skin, run a finger under an edge of the adhesive pad.
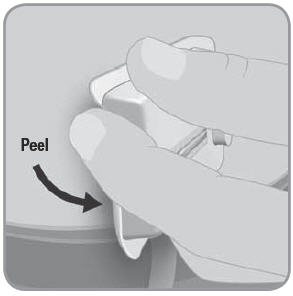
- F. Press lightly on the skin adjacent to the V-Go with one hand and peel the V-Go away from your skin with the other hand.
NOTE: After you remove the V-Go, the adhesive may leave behind a sticky residue. Use warm soapy water and roll your finger around the attachment site. This motion will remove the sticky adhesive residue. You could use a medical adhesive remover instead. Ask your doctor or healthcare professional to recommend an adhesive remover. Step 9: Dispose of the V-Go
Discard the used V-Go according to local disposal requirements.
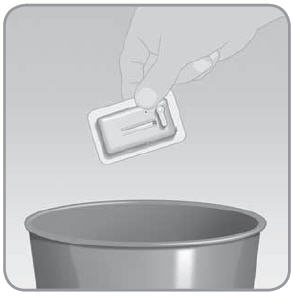
NOTE: When used as directed, the V-Go needle is never exposed. It comes out only when the device is attached to the skin. After use, the needle is retracted back into the V-Go and locked. Once retracted, the V-Go needle is completely contained/covered. Check to make sure the needle is retracted. If it is not, repeat Step 8C prior to disposal of the V-Go. SECTION 3: V-Go™ SAFETY INFORMATION
The V-Go is a safe and reliable device. As with many medical devices, you must be aware of safety-related issues to make sure that you are using the V-Go correctly. Always consult with your doctor or healthcare professional if you have any questions regarding the functions and operation of the V-Go.
Caution
Federal (United States) law restricts this device to sale by or on the order of a physician or properly licensed practitioner (prescription only).
Indication
V-Go 20:
The V-Go Disposable Insulin Delivery Device is indicated for continuous subcutaneous infusion of 20 Units of insulin in one 24-hour time period (0.83 U/hr) and on-demand bolus dosing in 2-Unit increments (up to 36 Units per one 24-hour time period) in adult patients requiring insulin.V-Go 30:
The V-Go Disposable Insulin Delivery Device is indicated for continuous subcutaneous infusion of 30 Units of insulin in one 24-hour time period (1.25 U/hr) and on-demand bolus dosing in 2-Unit increments (up to 36 Units per one 24-hour time period) in adult patients requiring insulin.V-Go 40:
The V-Go Disposable Insulin Delivery Device is indicated for continuous subcutaneous infusion of 40 Units of insulin in one 24-hour time period (1.67 U/hr) and on-demand bolus dosing in 2-Unit increments (up to 36 Units per one 24-hour time period) in adult patients requiring insulin.A U100 fast-acting insulin should be used with the V-Go. Humalog® (insulin lispro, rDNA origin) and NovoLog® (insulin aspart, rDNA origin) have been tested by Valeritas, Inc. and found to be safe for use in the V-Go Disposable Insulin Delivery Device.
Before using different insulin with the V-Go, you should check the insulin label to make sure it can be used with this device.
Healthcare Professional Dosing Considerations
When selecting a V-Go option, healthcare professionals should refer to their own experience when initiating continuous subcutaneous insulin infusion therapy with a patient. If unfamiliar, the healthcare professional should refer to insulin therapy guidelines from diabetes associations.
The following should be considered when initially prescribing the V-Go:
- Understand the total daily dose of insulin your patient is actually taking with their current insulin regimen versus what is being prescribed. Selecting the correct V-Go option may lessen the risk of hypoglycemia.
- It is common practice to reduce the total daily insulin dose when starting a patient on continuous subcutaneous insulin infusion therapy and this reduction should be considered when starting a patient on the V-Go.
Warnings
Insulin requirements
If you have to make regular adjustments or modifications to your basal rate during a 24-hour period, or if the amount of insulin used at meals requires adjustments of less than 2-Unit increments, use of the V-Go may result in hypoglycemia.
The following conditions may occur during insulin therapy with the V-Go.
- Hypoglycemia:
- – Intensive management of diabetes with too much insulin has been associated with an increase in the incidence of hypoglycemia (low blood sugar).
- Hyperglycemia and diabetic ketoacidosis (DKA):
- – Any insulin delivery interruption may result in hyperglycemia (high blood sugar) or the onset of diabetic ketoacidosis.
If you have a medical emergency while using the V-Go, call 911, your doctor, or go directly to the hospital.
Precautions
General
The following are a number of general precautions you should consider when using the V-Go™ Disposable Insulin Delivery Device.
The V-Go is magnetic resonance (MR) unsafe.
You should remove the V-Go before having an X-ray, MRI, or CT scan (or any similar test or procedure). Replace with a new V-Go after the test or procedure is completed.
You should monitor your blood glucose levels based on your doctor's or healthcare professional's recommendation. American Diabetes Association guidelines suggest that patients test blood glucose 3 or more times daily.
You should act quickly to respond to abnormal blood sugar levels.
- Notify your doctor or healthcare professional of any serious hypoglycemia or hyperglycemia. Tell your doctor or healthcare professional of any increased frequency in abnormally high or low blood glucose test results.
You should create a plan with your doctor or healthcare professional in case a problem occurs when you are unable to reach him or her for advice.
You should create a plan with your doctor or healthcare professional on how to manage your bolus (mealtime) dosing using the V-Go, including what to do if you lose count while bolus dosing or if you forget to take a bolus dose.
You should carry an emergency kit of insulin supplies, if instructed by your doctor or healthcare professional, in case you develop a problem with the V-Go that stops your insulin delivery. Tell a family member or friend where you keep your emergency kit items.
-
You should speak with your doctor or healthcare professional regarding what to have in the emergency kit, which often includes the following items:
- – Fast-acting glucose tablets
- – Blood glucose and urine ketone monitoring supplies
- – Back-up insulin, insulin syringe, and needles with directions from your doctor or healthcare professional regarding how much insulin to take
- – Dressing and adhesive
You should avoid exposing the V-Go to direct sunlight.
You should avoid exposure to extremely hot temperatures. Remove the V-Go prior to hot tub, whirlpool, or sauna use and replace with a new filled V-Go afterward.
You should check that the V-Go is securely in place during and after periods of increased physical activity. Check that the V-Go is securely in place if it has been exposed to water or gone under water to the depth of 3 feet, 3 inches (1 meter). The V-Go can go under water and will continue to work safely.
You should follow these precautions to help prevent problems with the V-Go placement:
- 1.
Never use insulin that appears cloudy. Cloudy insulin may be inactive. Do not use cloudy insulin with the V-Go.
- 2. Do not expose the insulin to extreme changes in temperature. Check the insulin package insert for temperature variation.
- 3. Practice aseptic technique when preparing, filling, and attaching the V-Go.
- 4. Check the adhesive site for redness, irritation, and inflammation when you remove a used V-Go and before you attach a new V-Go.
- 5. Change the application site each time you change the V-Go. See Section 2 (Part 3) Step 3. Changing the site will ensure proper absorption of insulin. The new site should be at least 1 inch away from the previous site.
- 6. Do not apply the V-Go to a site that has excess hair or is irritated, infected, or unhealthy for any reason. Consult with your doctor or healthcare professional about how to prepare and maintain these sites.
- 7. Avoid attaching the V-Go to sites that may interfere with your clothing, accessories, or car seatbelts.
- 8. Do not attach the V-Go to sites with rigorous movement and stretching due to exercise or job-related activities.
- 2. Do not expose the insulin to extreme changes in temperature. Check the insulin package insert for temperature variation.
Hypoglycemia
Low blood sugar is the most common side effect associated with any insulin, including the insulin delivered using the V-Go™. Symptoms of low blood sugar may vary and can happen suddenly.
To help prevent hypoglycemic episodes, follow these precautionary steps:
- 1.
Know the symptoms of hypoglycemia. Do not ignore these symptoms, no matter how mild they may be.
- 2. Always carry a fast-acting sugar replacement (such as candy, juice, or glucose tablets) in the event of a hypoglycemic episode.
- 3. The V-Go delivery rate can vary by up to +/- 10% from device to device. Even though the chance of this happening is remote, you should monitor your glucose level at least 3 times per day or as recommended by your doctor. Your doctor or healthcare professional may recommend specific times for you to check your blood glucose.
- 4. Check your blood glucose before driving or operating heavy machinery. Appropriate blood glucose levels are required to maintain alertness.
- 2. Always carry a fast-acting sugar replacement (such as candy, juice, or glucose tablets) in the event of a hypoglycemic episode.
IMPORTANT: If your glucose level falls below 70 mg/dL, you may be hypoglycemic (low blood sugar) and you should take immediate action to raise your blood glucose level. This may be done by taking glucose tablets, eating candy, drinking juice, or doing as your doctor or healthcare professional instructs. You should retest your blood glucose after 15 minutes and if it is still below 70 mg/dL continue to take steps to increase your blood glucose level until it reaches your normal level. Consult with your doctor or healthcare professional to understand how to best recognize and manage low blood glucose. Hyperglycemia and Diabetic Ketoacidosis (DKA)
To help prevent serious hyperglycemia (high blood sugar) and the possibility of diabetic ketoacidosis (DKA), follow these precautions:
- 1. Check your blood glucose frequently based on your doctor’s or healthcare professional's recommendation. Your doctor or healthcare professional may recommend specific times for you to check your blood glucose.
IMPORTANT: Nausea and vomiting are often the first signs of DKA. To avoid DKA, be prepared and act quickly. Don’t assume your blood glucose is high because you are under stress, have the flu, or miscalculated your last meal bolus. - 2.
Be sure you know when to test for ketones and when your doctor or healthcare professional expects you to call with results.
- 3. Know your blood glucose target ranges and when your doctor or healthcare professional expects you to report trouble. When your blood glucose is high, be prepared to administer insulin. If you suspect that the V-Go is not delivering insulin, refer to the Troubleshooting section of this manual on page 41.
- 4. Keep yourself well hydrated, especially during illness or exercise.
- 5. Do not treat DKA yourself. If you suspect DKA, contact your doctor or healthcare professional.
- 3. Know your blood glucose target ranges and when your doctor or healthcare professional expects you to report trouble. When your blood glucose is high, be prepared to administer insulin. If you suspect that the V-Go is not delivering insulin, refer to the Troubleshooting section of this manual on page 41.
Adverse Reactions
Site Infection/Abscesses
Infections at the infusion site may occur. Proper site preparation and frequent site rotation (refer to Section 2 (Part 3) Step 3A to 3D) can minimize infections. Remove the V-Go immediately if the area around the V-Go becomes sore, red, or swollen. Apply a new V-Go to a new, clean site away from the suspected infected area. Do not discontinue therapy without the advice of your doctor or healthcare professional.
SECTION 4: PRODUCT SPECIFICATIONS
EZ Fill Storage Conditions –
Unused Device Only-4°F (-20°C) to +140°F (+60°C)
20% to 90% relative humidityOperating Conditions +40°F (+5°C) to +99°F (+37°C)
20% to 90% relative humidity
Warm to room temperature to optimize fill qualityInsulin Each time before placing a new vial into the EZ Fill, check to make sure the insulin vial is not expired and that it will not expire during the time period that the vial will be used in the EZ Fill Duration of Use 30 fills Disposal Local disposal requirements V-Go™ Basal Delivery,
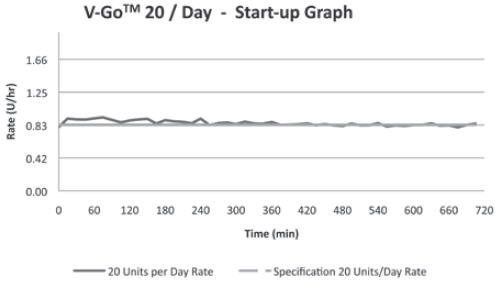
Continuous Subcutaneous20 Units/24 hr (0.83 U/hr)
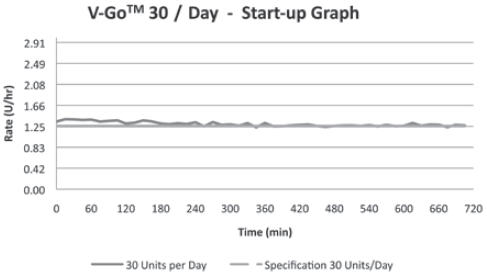
30 Units/24 hr (1.25 U/hr)
40 Units/24 hr (1.67 U/hr)Bolus Delivery, On-Demand Each push of the bolus delivery button provides 2 Units
Each V-Go contains 18 uses for a maximum of 36 Units per V-GoV-Go Minimum Capacity Reservoir 0.56 mL V-Go 20
0.66 mL V-Go 30
0.76 mL V-Go 40V-Go Size 2.4 x 1.3 x 0.5 inches
(6.1 cm x 3.3 cm x 1.3 cm)V-Go Weight 0.7 to 1.8 ounces
(20 to 50 grams)Storage Conditions –
Device Only+10°F (-12°C) to +140°F (+60°C)
20% to 90% relative humidity
Avoid prolonged exposure to the extremes of the storage temperature rangeStorage Conditions –
Filled with Humalog®The V-Go may be filled with Humalog for up to 24 hours prior to use if refrigerated or if left at room temperature Storage Conditions –
Filled with NovoLog®The V-Go may be filled with NovoLog for up to 5 days prior to use if refrigerated
The V-Go may be filled with NovoLog for up to 3 days prior to use if left at room temperatureOperating Conditions +40°F (+5°C) to +99°F (+37°C)
20% to 90% relative humidity
Do not expose the V-Go to temperatures over 99°F for prolonged periods of time (beyond 14 hours) as this may lead to degradation of the insulin (check the insulin manufacturer's instructions for use for temperature details) and may cause the V-Go to run at a basal rate slightly greater than the specified rangeOperating Atmospheric Pressure 697 hPa to 1,013 hPa
(Equivalent to altitudes up to 10,000 feet [3,048 meters])Duration of Use 24 hr Bolus Delivery Accuracy
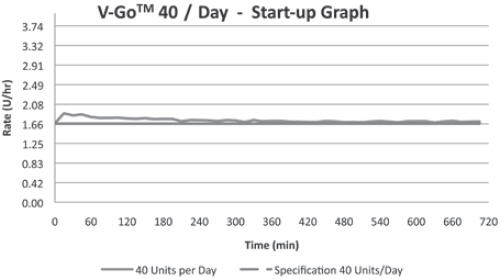
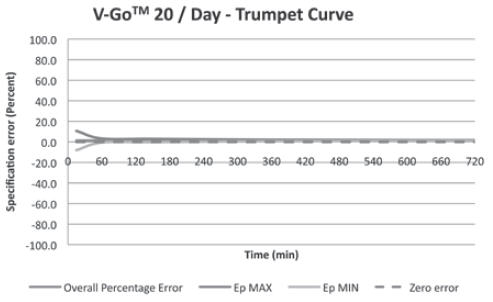
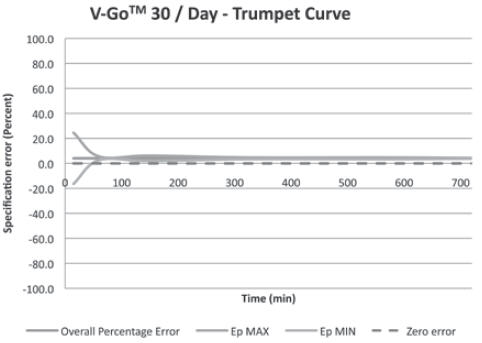
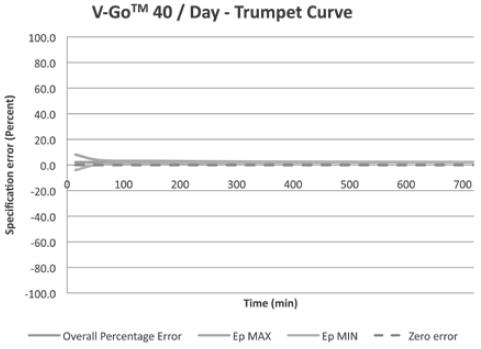
(For all 3 V-Go options)+/-10% Basal Delivery Accuracy
(For all 3 V-Go options)+/-10% V-Go Power Source Mechanical Enclosure Protection Protects against the effects of submersion at depths up to 3 feet, 3 inches (1 meter) for 24 hours.
(IPX8)Needle 4.6-mm, 30-gauge needle with sharps injury prevention features and floating needle technology Disposal Local disposal requirements SECTION 5: V-Go™ USER ASSISTANCE INFORMATION
Valeritas Customer Care
You may have questions or concerns when you use a new product. Please contact us for advice or assistance. A trained staff member will help you with any questions on how to fill, apply, or use the V-Go.
For specific treatment questions, information on insulin, or medical advice, call your doctor or healthcare professional.
If you have any questions regarding the operation of the V-Go or EZ Fill, speak to a Valeritas Customer Care Representative 24/7 at 1-866-881-1209.
Customers can visit the Valeritas website (www.valeritas.com) for additional information about disposable insulin delivery with the V-Go.
If you have a medical emergency while using the V-Go, call 911, your doctor, or go directly to the hospital.
SECTION 6: TROUBLESHOOTING/FREQUENTLY ASKED QUESTIONS
How do I know how much insulin to put into the V-Go?
You always fill the V-Go completely. The V-Go comes in 3 options and your doctor or healthcare professional will select the option you need.
Why does insulin leak from the EZ Fill?
Check to make sure a V-Go is placed in the EZ Fill before a new insulin vial is placed into the insulin vial holder. This may reduce any insulin leak that can happen when extra pressure is built up in the vial upon initial puncture. Also, make sure the plug is properly inserted into the EZ Fill between fills.
It looks like the V-Go has not filled completely.
There are 5 reasons the V-Go may not fill completely:
- 1.
The insulin vial is not completely pushed down into the insulin vial holder.
- If this is the case, push the vial firmly straight down into the insulin vial holder until you feel it attach and cannot push it further.
- 2. You have run out of insulin in your vial. You have to switch vials before you can fill the rest of the V-Go.
- If this is the case, keep the V-Go in the EZ Fill drawer. Remove the insulin vial from the EZ Fill insulin vial holder, replace with a new vial, and refill the V-Go.
- 3. You did not completely pull the plunger to its full upright position.
- If this is the case, slowly pull the plunger up completely and repeat the filling process for the V-Go.
- 4. You have pushed down the plunger too quickly, which turned the insulin foamy. The foam could become an air bubble larger than a grain of rice.
- If this is the case, the EZ Fill cannot correct this error at this time. Set the V-Go aside for use on the next day and start over with a new V-Go.
- – The V-Go with the foamy insulin should be stored with the insulin port up. Before using this V-Go, refill it completely with insulin. See Section 2 (Part 2) Step 4A to 4F. Refer to the V-Go storage conditions in Section 4.
- 5. The EZ Fill drawer may not be completely closed.
- If this is the case, check again to ensure the drawer is pushed all the way closed.
In any of these situations follow Step 4A to 4F to make sure you have a complete fill.
I am looking in the viewing window while pushing the plunger down, but I don’t see the V-Go™ filling.
There are 2 reasons you may not see any insulin fill the V-Go.
- 1.
You have run out of insulin in your vial. You have to switch vials before you can fill the rest of the V-Go.
- If this is the case, keep the V-Go in the EZ Fill drawer. Remove the insulin vial from the EZ Fill insulin vial holder, replace with a new vial, and refill the V-Go.
- 2. You did not completely shut the EZ Fill drawer.
- Grip the ribbed drawer handle and push the drawer completely into the EZ Fill. The ribbed drawer handle should not stick out past the bottom of the EZ Fill.
If there is insulin in the vial and the drawer is completely shut, try to fill a new V-Go. If you are unable to fill a new V-Go, speak to a Valeritas Customer Care Representative at 1-866-881-1209.
There are bubbles in the insulin vial.
The most common reason for bubbles in the insulin vial is filling more than 1 V-Go at a time. The EZ Fill is designed to fill only 1 V-Go per day.
It is harder to push the plunger when it gets closer to the downward position.
This is normal. When the V-Go is full or close to full, you should expect to push a little harder. You may need a little extra pressure to push the plunger completely down.
What happens if I do not pull the plunger completely up when I fill the V-Go?
If the plunger is pulled only part of the way up during the fill process, you will get only a partial fill of the V-Go. You will see air bubbles in the V-Go viewing window. Keep the V-Go in the EZ Fill drawer and slowly pull the plunger up completely again. Make sure the plunger is completely up. Slowly and steadily push the plunger back down over about 25 seconds until it is in the complete down position.
I tried to pull the plunger up and it snapped back down. What should I do?
Try slowly pulling the plunger up again, but count the full 5 seconds before releasing or reversing the direction of the plunger. The plunger snaps back down if you try to pull the plunger up and release it in less than 5 seconds.
After I place the V-Go into the EZ Fill drawer, what happens if the plunger is already in the up position?
You can still fill the V-Go. Hold the EZ Fill upwards and slowly and steadily push the plunger down over about 25 seconds until it is in the complete down position. As you push the plunger down, check to make sure insulin enters the V-Go. Repeat the plunger cycle if no insulin entered the V-Go or if additional insulin is required to fill the V-Go. See Section 2 (Part 2) Step 4A to 4F.
I did not apply the V-Go directly after filling. Do I have to throw it away?
It is preferable to apply the V-Go immediately after filling. However, refer to the V-Go storage conditions in Section 4 and the specific insulin’s storage conditions for more information.
I believe an item is missing or appears to be damaged.
If you suspect an item is damaged, do not use the damaged piece. Replace this with a new one (V-Go or EZ Fill). EZ Fill replacements can be requested from a Valeritas Customer Care Representative at 1-866-881-1209.
I believe I have broken the EZ Fill. How do I get another one to fill the V-Go™?
EZ Fill replacements can be requested from a Valeritas Customer Care Representative at 1-866-881-1209. You will be asked to return the unusable EZ Fill. Valeritas Customer Care will provide you with further details.
The inner package for the V-Go is torn or damaged.
If you suspect the package containing the V-Go has been torn or damaged, do not use this V-Go. Replace with a new V-Go.
After I removed the button cover, the cover pin was not attached to the button cover and is still inside the V-Go.
If the cover pin is inside the V-Go, the V-Go will not work correctly. Try to remove the pin manually; otherwise discard this V-Go and start over with a new V-Go.
It is difficult to press down on the needle button.
Be sure you are pressing the raised bump of the needle button. If the needle button does not move when pressed, the needle button may have already been activated. Do not use this V-Go. Replace with a new V-Go.
The needle button will not stay in the down position.
The needle button must be pressed down fully to be locked into place. If you cannot get the button to stay down, do not use this V-Go. Replace with a new V-Go.
The V-Go won’t remain securely attached to my skin for the duration of use, which sometimes causes poking of the needle.
If the V-Go will not remain secure, replace with a new V-Go.
When replacing the V-Go, please review Section 2 (Part 3) Step 3A to 3D.
Do not touch the adhesive pad before you attach the V-Go to your skin. Thoroughly clean the site with an alcohol swab. Let the site dry before attaching the V-Go. Many lotions and ointments may keep the V-Go from sticking to your skin.
Be sure that you have properly attached the V-Go to a flat surface on your body.
If the site is covered with hair, this could also affect whether the V-Go sticks. You may shave the area to help the V-Go attach to your skin.
When I went to give myself a bolus dose of insulin, the bolus delivery button was already popped out.
If the bolus delivery button was already popped out, the bolus ready button was already activated. Press the bolus delivery button to deliver 2 Units of insulin when the next bolus dose is needed.
The bolus delivery button will not pop out.
First check that you are pressing the bolus ready button fully. If the bolus delivery button fails to pop out after you activate the bolus ready button, you have used all of the available insulin for bolus dosing (36 Units or 18 uses per 24 hours). Replace this V-Go with a new V-Go before your next meal. Plan your day so that you have enough insulin for each meal.
What should I do if I forget the number of Units I have delivered when bolus dosing or if I forget to take a bolus dose?
Follow the plan you created with your doctor or healthcare professional for such situations. If you have not created a plan already, consult your doctor or healthcare professional for guidance.
The area of skin around the V-Go is red and sore to the touch.
Skin may be irritated by the adhesive pad on the V-Go. There are a number of skin barrier products available to help prevent irritation and treat sensitivity problems. Contact your doctor or healthcare professional for specific products and recommendations.
Infections at the site may also occur. If you experience redness, irritation, or inflammation around the V-Go (specifically the end closest to the needle), immediately replace the V-Go with a new V-Go on a new body site. Contact your doctor or healthcare professional for the best way to treat infection and irritation, especially if the inflamed site appears bigger than a dime.
I dropped the V-Go™.
Check to see if there is any damage to the V-Go. If you suspect that the V-Go was damaged or became dirty as a result of the fall, do not use this one. Replace with a new V-Go.
I dropped the EZ Fill.
Check to see if there is any damage to the EZ Fill or the insulin vial, if it was attached. If the insulin vial came out of the EZ Fill, wipe the top of the vial with an alcohol swab and watch closely for leaks when you place the vial back into the EZ Fill. If insulin is leaking, replace the insulin vial with a new one. If you suspect that the EZ Fill was damaged, speak to a Valeritas Customer Care Representative at 1-866-881-1209.
The V-Go was exposed to water. Is this a problem?
The V-Go can go under water up to 3 feet, 3 inches (1 meter), and will continue to work safely. You should check to see that it stays in place. Depending on the cleanliness of the water, you may be at an increased risk for an infection at the needle infusion site.
Can I swim, scuba dive, shower, or bathe while wearing the V-Go?
The V-Go has been successfully tested in water to depths of 3 feet, 3 inches (1 meter) for 24 hours, and therefore can be worn during normal daily activities, such as showering and bathing as well as activities near the surface of the water, such as swimming.
The V-Go has not been tested and therefore should not be used in the following conditions:
- Water activities below 3 feet, 3 inches (1 meter), such as scuba diving.
- Extremely hot water temperatures, such as hot tubs or whirlpools.
Remove the V-Go prior to scuba diving, hot tub use, or whirlpool use, and replace with a new filled V-Go afterward.
Can I wear the V-Go for longer than 24 hours – for example, if I sleep an extra hour on the weekend?
The V-Go has been designed for 24-hour wear. You should change it at the same time each day. After 24 hours, the V-Go may no longer deliver a continuous preset basal rate of insulin.
How do I travel with the V-Go?
Use the same precautions that you would when traveling with other insulin supplies. Speak with your doctor or healthcare professional about extra precautions you may need to take while traveling.
When traveling with the EZ Fill, with an insulin vial attached, it should be refrigerated at all times. Empty V-Go devices do not require refrigeration. They should be stored according to the storage conditions in Section 4. For information on storing filled V-Go devices refer to the V-Go storage conditions in Section 4.
Can I wear the V-Go on an airplane?
Yes, the V-Go can safely be worn on an airplane.
Should I wear the V-Go to bed?
Yes, you should wear the V-Go for a full 24 hours, even while you sleep.
The needle will not retract back into the V-Go.
Try to slide and press the needle release button again. See Section 2 (Part 3) Step 8C. If the needle still will not retract into the V-Go, dispose of the V-Go in a sharps container.
SECTION 7: GLOSSARY AND PACKAGING SYMBOLS
Aseptic technique: This process helps keep the V-Go™ and EZ Fill devices clean during preparation, filling, and application. Following this technique requires handwashing and wiping the application site with an alcohol swab. It may involve wiping the V-Go device with an alcohol swab if the device comes into contact with anything else.
Basal rate: The basal rate is the amount of insulin delivered at a preset rate by the V-Go over 24 hours. Your basal rate is the amount of insulin required to maintain your target glucose values when you are not eating. Basal rate may also be referred to as background, continuous, or long-acting insulin.
Blood glucose levels: Blood glucose levels are the measure of how much sugar is in the blood.
Bolus: A bolus is an amount of insulin delivered at one time. A bolus is usually taken before a meal to cover your body's insulin needs. A bolus of insulin can also be taken when blood glucose levels are abnormally high. Bolus may also be referred to as mealtime, prandial, short-acting, or rapid-acting insulin.
Diabetes mellitus: Diabetes is a disease in which the body cannot maintain its own healthy blood glucose levels. Either the body cannot produce enough insulin or the body cannot properly use insulin. There are two main types of diabetes:
- Type 1 diabetes: In Type 1 diabetes, the body does not produce enough insulin. People with Type 1 diabetes must use insulin to regulate their blood glucose levels.
- Type 2 diabetes: In Type 2 diabetes, the body does not produce enough insulin or use it properly. People with Type 2 diabetes can usually regulate their blood glucose levels by following an individual meal plan, exercising, and taking certain medicines, including insulin, if needed. It usually occurs in people 40 years or older.
Diabetic ketoacidosis (DKA): DKA occurs when the blood glucose level is elevated and the insulin level is low. The body does not have enough insulin to help the glucose enter the cells. Glucose in the cells is used for energy. During this situation, the body begins to burn muscle and fat for energy. A waste product of fat burning is ketones. Ketones accumulate in the blood and are passed through the urine and lungs. This condition can be identified by urine and/or blood tests. DKA usually requires hospitalization and can lead to a life-threatening situation if not promptly treated.
Glucose: Glucose, a sugar, is a main way the body takes in carbohydrates. Glucose is the body’s most important source of energy. It is produced from digested food, by the normal action of the liver. Glucose is carried by the blood throughout the body.
Hyperglycemia: Hyperglycemia or high blood sugar occurs when blood glucose levels rise above normal (approximately 250 mg/dL). Hyperglycemia is the result of the body not having enough insulin or not being able to use insulin to process glucose.
Hypoglycemia: Hypoglycemia or low blood sugar occurs when blood glucose levels drop below normal (approximately 70 mg/dL). Hypoglycemia may result from too much insulin and/or exercising more than usual.
Infusion site: The infusion site is the place on the body where the V-Go is attached, specifically the end of the V-Go where the needle is inserted under the skin.
Insulin: Insulin is a hormone produced by the pancreas. Insulin is needed by the body to regulate the production and use of glucose.
Ketones: Ketones, or ketone bodies, are substances produced by normal liver activity, and used by muscle tissue. When blood glucose levels are elevated, the body's normal process is unbalanced and ketones can accumulate in the blood, pass through the urine, and ultimately result in diabetic ketoacidosis (DKA).
Subcutaneous: Subcutaneous means beneath the layer of the skin. The V-Go infusion needle delivers insulin subcutaneously. Subcutaneous injections are not the same thing as intravenous (IV) or intramuscular (IM) injections.
-
V-Go 20 Commercial Kit
8560-9400-03
V-Go™
DISPOSABLE INSULIN DELIVERY20
Kit Contents:
- Thirty (30) sterile, nonpyrogenic, single-use devices to be used over 24 hours (nonreusable)
- One (1) sterile, nonpyrogenic filling device designed for 30 fills with one (1) plug and one (1) cap
- One (1) V-Go Instructions for Patient Use insert
- One (1) V-Go Quick Reference Guide insert
Insulin is not included in this kit.
Dispense as one (1) kit. Do not break apart.
PHARMACIST
Dispense two (2) vials of fast-acting insulin2 with the V-Go 20
Patient counseling materials included in kit1-866-881-1209
www.go-vgo.comvaleritas

- 2 See Instructions for Patient Use (Section 3) for insulins tested with the V-Go
-
V-Go 30 Commercial Kit
8560-9400-02
V-Go™
DISPOSABLE INSULIN DELIVERY30
Kit Contents:
- Thirty (30) sterile, nonpyrogenic, single-use devices to be used over 24 hours (nonreusable)
- One (1) sterile, nonpyrogenic filling device designed for 30 fills with one (1) plug and one (1) cap
- One (1) V-Go Instructions for Patient Use insert
- One (1) V-Go Quick Reference Guide insert
Insulin is not included in this kit.
Dispense as one (1) kit. Do not break apart.
PHARMACIST
Dispense three (3) vials of fast-acting insulin3 with the V-Go 30
Patient counseling materials included in kit1-866-881-1209
www.go-vgo.comvaleritas

- 3 See Instructions for Patient Use (Section 3) for insulins tested with the V-Go
-
V-Go 40 Commercial Kit
8560-9400-01
V-Go™
DISPOSABLE INSULIN DELIVERY40
Kit Contents:
- Thirty (30) sterile, nonpyrogenic, single-use devices to be used over 24 hours (nonreusable)
- One (1) sterile, nonpyrogenic filling device designed for 30 fills with one (1) plug and one (1) cap
- One (1) V-Go Instructions for Patient Use insert
- One (1) V-Go Quick Reference Guide insert
Insulin is not included in this kit.
Dispense as one (1) kit. Do not break apart.
PHARMACIST
Dispense three (3) vials of fast-acting insulin4 with the V-Go 40
Patient counseling materials included in kit1-866-881-1209
www.go-vgo.comvaleritas

- 4 See Instructions for Patient Use (Section 3) for insulins tested with the V-Go
-
INGREDIENTS AND APPEARANCE
V-GO DISPOSABLE INSULIN DELIVERY DEVICE
pump, infusion, insulinProduct Information Product Type PRESCRIPTION MEDICAL DEVICE Item Code (Source) NHRIC:8560-9400 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:8560-9400-03 30 in 1 BOX 2 NHRIC:8560-9400-02 30 in 1 BOX 3 NHRIC:8560-9400-01 30 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date premarket notification K100504 02/06/2012 Labeler - Valeritas, Inc (786236781)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 Rx Only
Rx Only
 Store at
Store at
 Unless opened or damaged, contents are sterile
Unless opened or damaged, contents are sterile
 Do not reuse
Do not reuse
 Dispose of after 30 fills
Dispose of after 30 fills
 Use by
Use by
 Before using contents, read the Instructions for Patient Use
Before using contents, read the Instructions for Patient Use
 Lot code
Lot code