INTRON A- interferon alfa-2b kit INTRON A- interferon alfa-2b injection, solution
INTRON A by
Drug Labeling and Warnings
INTRON A by is a Prescription medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING
Alpha interferons, including INTRON® A, cause or aggravate fatal or life-threatening neuropsychiatric, autoimmune, ischemic, and infectious disorders. Patients should be monitored closely with periodic clinical and laboratory evaluations. Patients with persistently severe or worsening signs or symptoms of these conditions should be withdrawn from therapy. In many but not all cases these disorders resolve after stopping INTRON A therapy. See WARNINGS and ADVERSE REACTIONS.
-
DESCRIPTION
INTRON® A (Interferon alfa-2b) for intramuscular, subcutaneous, intralesional, or intravenous Injection is a purified sterile recombinant interferon product.
INTRON A recombinant for Injection has been classified as an alpha interferon and is a water-soluble protein with a molecular weight of 19,271 daltons produced by recombinant DNA techniques. It is obtained from the bacterial fermentation of a strain of Escherichia coli bearing a genetically engineered plasmid containing an interferon alfa-2b gene from human leukocytes. The fermentation is carried out in a defined nutrient medium containing the antibiotic tetracycline hydrochloride at a concentration of 5 to 10 mg/L; the presence of this antibiotic is not detectable in the final product. The specific activity of interferon alfa-2b, recombinant is approximately 2.6 × 108 IU/mg protein as measured by the HPLC assay.
Powder for Injection Vial Strength
Million IUmL Diluent Final Concentration after Reconstitution million IU/mL* mg INTRON A† per vial Route of Administration - * Each mL also contains 20 mg glycine, 2.3 mg sodium phosphate dibasic, 0.55 mg sodium phosphate monobasic, and 1.0 mg human albumin.
- † Based on the specific activity of approximately 2.6 × 108 IU/mg protein, as measured by HPLC assay.
10 1 10 0.038 IM, SC, IV, IL 18 1 18 0.069 IM, SC, IV 50 1 50 0.192 IM, SC, IV Prior to administration, the INTRON A Powder for Injection is to be reconstituted with the provided Diluent for INTRON A (Sterile Water for Injection USP) (see DOSAGE AND ADMINISTRATION). INTRON A Powder for Injection is a white to cream-colored powder.
Solution Vials for Injection Vial Strength Concentration* mg INTRON A† per vial Route of Administration - * Each mL contains 7.5 mg sodium chloride, 1.8 mg sodium phosphate dibasic, 1.3 mg sodium phosphate monobasic, 0.1 mg edetate disodium, 0.1 mg polysorbate 80, and 1.5 mg m-cresol as a preservative.
- † Based on the specific activity of approximately 2.6 × 108 IU/mg protein as measured by HPLC assay.
- ‡ This is a multidose vial which contains a total of 22.8 million IU of interferon alfa-2b, recombinant per 3.8 mL in order to provide the delivery of six 0.5-mL doses, each containing 3 million IU of INTRON A (for a label strength of 18 million IU).
- § This is a multidose vial which contains a total of 32.0 million IU of interferon alfa-2b, recombinant per 3.2 mL in order to provide the delivery of five 0.5-mL doses, each containing 5 million IU of INTRON A (for a label strength of 25 million IU).
18‡ MIU multidose 3 million IU/0.5 mL 0.088 IM, SC 25§ MIU multidose 5 million IU/0.5 mL 0.123 IM, SC, IL These packages do not require reconstitution prior to administration (see DOSAGE AND ADMINISTRATION). INTRON A Solution for Injection is a clear, colorless solution.
-
CLINICAL PHARMACOLOGY
General
The interferons are a family of naturally occurring small proteins and glycoproteins with molecular weights of approximately 15,000 to 27,600 daltons produced and secreted by cells in response to viral infections and to synthetic or biological inducers.
Preclinical Pharmacology
Interferons exert their cellular activities by binding to specific membrane receptors on the cell surface. Once bound to the cell membrane, interferons initiate a complex sequence of intracellular events. In vitro studies demonstrated that these include the induction of certain enzymes, suppression of cell proliferation, immunomodulating activities such as enhancement of the phagocytic activity of macrophages and augmentation of the specific cytotoxicity of lymphocytes for target cells, and inhibition of virus replication in virus-infected cells.
In a study using human hepatoblastoma cell line HB 611, the in vitro antiviral activity of alpha interferon was demonstrated by its inhibition of hepatitis B virus (HBV) replication.
The correlation between these in vitro data and the clinical results is unknown. Any of these activities might contribute to interferon's therapeutic effects.
Pharmacokinetics
The pharmacokinetics of INTRON® A were studied in 12 healthy male volunteers following single doses of 5 million IU/m2 administered intramuscularly, subcutaneously, and as a 30-minute intravenous infusion in a crossover design.
The mean serum INTRON A concentrations following intramuscular and subcutaneous injections were comparable. The maximum serum concentrations obtained via these routes were approximately 18 to 116 IU/mL and occurred 3 to 12 hours after administration. The elimination half-life of INTRON A following both intramuscular and subcutaneous injections was approximately 2 to 3 hours. Serum concentrations were undetectable by 16 hours after the injections.
After intravenous administration, serum INTRON A concentrations peaked (135-273 IU/mL) by the end of the 30-minute infusion, then declined at a slightly more rapid rate than after intramuscular or subcutaneous drug administration, becoming undetectable 4 hours after the infusion. The elimination half-life was approximately 2 hours.
Urine INTRON A concentrations following a single dose (5 million IU/m2) were not detectable after any of the parenteral routes of administration. This result was expected since preliminary studies with isolated and perfused rabbit kidneys have shown that the kidney may be the main site of interferon catabolism.
There are no pharmacokinetic data available for the intralesional route of administration.
Serum Neutralizing Antibodies
In INTRON A-treated patients tested for antibody activity in clinical trials, serum anti-interferon neutralizing antibodies were detected in 0% (0/90) of patients with hairy cell leukemia, 0.8% (2/260) of patients treated intralesionally for condylomata acuminata, and 4% (1/24) of patients with AIDS-Related Kaposi's Sarcoma. Serum neutralizing antibodies have been detected in less than 3% of patients treated with higher INTRON A doses in malignancies other than hairy cell leukemia or AIDS-Related Kaposi's Sarcoma. The clinical significance of the appearance of serum anti-interferon neutralizing activity in these indications is not known.
Serum anti-interferon neutralizing antibodies were detected in 7% (12/168) of patients either during treatment or after completing 12 to 48 weeks of treatment with 3 million IU TIW of INTRON A therapy for chronic hepatitis C and in 13% (6/48) of patients who received INTRON A therapy for chronic hepatitis B at 5 million IU QD for 4 months, and in 3% (1/33) of patients treated at 10 million IU TIW. Serum anti-interferon neutralizing antibodies were detected in 9% (5/53) of pediatric patients who received INTRON A therapy for chronic hepatitis B at 6 million IU/m2 TIW. Among all chronic hepatitis B or C patients, pediatrics and adults with detectable serum neutralizing antibodies, the titers detected were low (22/24 with titers less than or equal to 1:40 and 2/24 with titers less than or equal to 1:160). The appearance of serum anti-interferon neutralizing activity did not appear to affect safety or efficacy.
Hairy Cell Leukemia
In clinical trials in patients with hairy cell leukemia, there was depression of hematopoiesis during the first 1 to 2 months of INTRON A treatment, resulting in reduced numbers of circulating red and white blood cells, and platelets. Subsequently, both splenectomized and nonsplenectomized patients achieved substantial and sustained improvements in granulocytes, platelets, and hemoglobin levels in 75% of treated patients and at least some improvement (minor responses) occurred in 90%. INTRON A treatment resulted in a decrease in bone marrow hypercellularity and hairy cell infiltrates. The hairy cell index (HCI), which represents the percent of bone marrow cellularity times the percent of hairy cell infiltrate, was greater than or equal to 50% at the beginning of the study in 87% of patients. The percentage of patients with such an HCI decreased to 25% after 6 months and to 14% after 1 year. These results indicate that even though hematologic improvement had occurred earlier, prolonged INTRON A treatment may be required to obtain maximal reduction in tumor cell infiltrates in the bone marrow.
The percentage of patients with hairy cell leukemia who required red blood cell or platelet transfusions decreased significantly during treatment and the percentage of patients with confirmed and serious infections declined as granulocyte counts improved. Reversal of splenomegaly and of clinically significant hypersplenism was demonstrated in some patients.
A study was conducted to assess the effects of extended INTRON A treatment on duration of response for patients who responded to initial therapy. In this study, 126 responding patients were randomized to receive additional INTRON A treatment for 6 months or observation for a comparable period, after 12 months of initial INTRON A therapy. During this 6-month period, 3% (2/66) of INTRON A-treated patients relapsed compared with 18% (11/60) who were not treated. This represents a significant difference in time to relapse in favor of continued INTRON A treatment (P=0.006/0.01, Log Rank/Wilcoxon). Since a small proportion of the total population had relapsed, median time to relapse could not be estimated in either group. A similar pattern in relapses was seen when all randomized treatment, including that beyond 6 months, and available follow-up data were assessed. The 15% (10/66) relapses among INTRON A patients occurred over a significantly longer period of time than the 40% (24/60) with observation (P=0.0002/0.0001, Log Rank/Wilcoxon). Median time to relapse was estimated, using the Kaplan-Meier method, to be 6.8 months in the observation group but could not be estimated in the INTRON A group.
Subsequent follow-up with a median time of approximately 40 months demonstrated an overall survival of 87.8%. In a comparable historical control group followed for 24 months, overall median survival was approximately 40%.
Malignant Melanoma
The safety and efficacy of INTRON A was evaluated as adjuvant to surgical treatment in patients with melanoma who were free of disease (post surgery) but at high risk for systemic recurrence. These included patients with lesions of Breslow thickness greater than 4 mm, or patients with lesions of any Breslow thickness with primary or recurrent nodal involvement. In a randomized, controlled trial in 280 patients, 143 patients received INTRON A therapy at 20 million IU/m2 intravenously five times per week for 4 weeks (induction phase) followed by 10 million IU/m2 subcutaneously three times per week for 48 weeks (maintenance phase). In the clinical trial, the median daily INTRON A dose administered to patients was 19.1 million IU/m2 during the induction phase and 9.1 million IU/m2 during the maintenance phase. INTRON A therapy was begun less than or equal to 56 days after surgical resection. The remaining 137 patients were observed.
INTRON A therapy produced a significant increase in relapse-free and overall survival. Median time to relapse for the INTRON A-treated patients versus observation patients was 1.72 years versus 0.98 years (P<0.01, stratified Log Rank). The estimated 5-year relapse-free survival rate, using the Kaplan-Meier method, was 37% for INTRON A-treated patients versus 26% for observation patients. Median overall survival time for INTRON A-treated patients versus observation patients was 3.82 years versus 2.78 years (P=0.047, stratified Log Rank). The estimated 5-year overall survival rate, using the Kaplan-Meier method, was 46% for INTRON A-treated patients versus 37% for observation patients.
In a second study of 642 resected high-risk melanoma patients, subjects were randomized equally to one of three groups: high-dose INTRON A therapy for 1 year (same schedule as above), low-dose INTRON A therapy for 2 years (3 MU/d TIW SC), and observation. Consistent with the earlier trial, high-dose INTRON A therapy demonstrated an improvement in relapse-free survival (3-year estimated RFS 48% versus 41%; median RFS 2.4 versus 1.6 years, P=not significant). Relapse-free survival in the low-dose INTRON A arm was similar to that seen in the observation arm. Neither high-dose nor low-dose INTRON A therapy showed a benefit in overall survival as compared to observation in this study.
Follicular Lymphoma
The safety and efficacy of INTRON A in conjunction with CHVP, a combination chemotherapy regimen, was evaluated as initial treatment in patients with clinically aggressive, large tumor burden, Stage III/IV follicular Non-Hodgkin's Lymphoma. Large tumor burden was defined by the presence of any one of the following: a nodal or extranodal tumor mass with a diameter of greater than 7 cm; involvement of at least three nodal sites (each with a diameter of greater than 3 cm); systemic symptoms; splenomegaly; serous effusion, orbital or epidural involvement; ureteral compression; or leukemia.
In a randomized, controlled trial, 130 patients received CHVP therapy and 135 patients received CHVP therapy plus INTRON A therapy at 5 million IU subcutaneously three times weekly for the duration of 18 months. CHVP chemotherapy consisted of cyclophosphamide 600 mg/m2, doxorubicin 25 mg/m2, and teniposide (VM-26) 60 mg/m2, administered intravenously on Day 1 and prednisone at a daily dose of 40 mg/m2 given orally on Days 1 to 5. Treatment consisted of six CHVP cycles administered monthly, followed by an additional six cycles administered every 2 months for 1 year. Patients in both treatment groups received a total of 12 CHVP cycles over 18 months.
The group receiving the combination of INTRON A therapy plus CHVP had a significantly longer progression-free survival (2.9 years versus 1.5 years, P=0.0001, Log Rank test). After a median follow-up of 6.1 years, the median survival for patients treated with CHVP alone was 5.5 years while median survival for patients treated with CHVP plus INTRON A therapy had not been reached (P=0.004, Log Rank test). In three additional published, randomized, controlled studies of the addition of interferon alpha to anthracycline-containing combination chemotherapy regimens,1-3 the addition of interferon alpha was associated with significantly prolonged progression-free survival. Differences in overall survival were not consistently observed.
Condylomata Acuminata
Condylomata acuminata (venereal or genital warts) are associated with infections of the human papilloma virus (HPV). The safety and efficacy of INTRON A in the treatment of condylomata acuminata were evaluated in three controlled double-blind clinical trials. In these studies, INTRON A doses of 1 million IU per lesion were administered intralesionally three times a week (TIW), in less than or equal to 5 lesions per patient for 3 weeks. The patients were observed for up to 16 weeks after completion of the full treatment course.
INTRON A treatment of condylomata was significantly more effective than placebo, as measured by disappearance of lesions, decreases in lesion size, and by an overall change in disease status. Of 192 INTRON A-treated patients and 206 placebo-treated patients who were evaluable for efficacy at the time of best response during the course of the study, 42% of INTRON A patients versus 17% of placebo patients experienced clearing of all treated lesions. Likewise, 24% of INTRON A patients versus 8% of placebo patients experienced marked (75% to less than 100%) reduction in lesion size, 18% versus 9% experienced moderate (50% to 75%) reduction in lesion size, 10% versus 42% had a slight (less than 50%) reduction in lesion size, 5% versus 24% had no change in lesion size, and 0% versus 1% experienced exacerbation (P<0.001).
In one of these studies, 43% (54/125) of patients in whom multiple (less than or equal to 3) lesions were treated experienced complete clearing of all treated lesions during the course of the study. Of these patients, 81% remained cleared 16 weeks after treatment was initiated.
Patients who did not achieve total clearing of all their treated lesions had these same lesions treated with a second course of therapy. During this second course of treatment, 38% to 67% of patients had clearing of all treated lesions. The overall percentage of patients who had cleared all their treated lesions after two courses of treatment ranged from 57% to 85%.
INTRON A-treated lesions showed improvement within 2 to 4 weeks after the start of treatment in the above study; maximal response to INTRON A therapy was noted 4 to 8 weeks after initiation of treatment.
The response to INTRON A therapy was better in patients who had condylomata for shorter durations than in patients with lesions for a longer duration.
Another study involved 97 patients in whom three lesions were treated with either an intralesional injection of 1.5 million IU of INTRON A per lesion followed by a topical application of 25% podophyllin, or a topical application of 25% podophyllin alone. Treatment was given once a week for 3 weeks. The combined treatment of INTRON A and podophyllin was shown to be significantly more effective than podophyllin alone, as determined by the number of patients whose lesions cleared. This significant difference in response was evident after the second treatment (Week 3) and continued through 8 weeks post-treatment. At the time of the patient's best response, 67% (33/49) of the INTRON A- and podophyllin-treated patients had all three treated lesions clear while 42% (20/48) of the podophyllin-treated patients had all three clear (P=0.003).
AIDS-Related Kaposi's Sarcoma
The safety and efficacy of INTRON A in the treatment of Kaposi's Sarcoma (KS), a common manifestation of the Acquired Immune Deficiency Syndrome (AIDS), were evaluated in clinical trials in 144 patients.
In one study, INTRON A doses of 30 million IU/m2 were administered subcutaneously three times per week (TIW) to patients with AIDS-Related KS. Doses were adjusted for patient tolerance. The average weekly dose delivered in the first 4 weeks was 150 million IU; at the end of 12 weeks this averaged 110 million IU/week; and by 24 weeks averaged 75 million IU/week.
Forty-four percent of asymptomatic patients responded versus 7% of symptomatic patients. The median time to response was approximately 2 months and 1 month, respectively, for asymptomatic and symptomatic patients. The median duration of response was approximately 3 months and 1 month, respectively, for the asymptomatic and symptomatic patients. Baseline T4/T8 ratios were 0.46 for responders versus 0.33 for nonresponders.
In another study, INTRON A doses of 35 million IU were administered subcutaneously, daily (QD), for 12 weeks. Maintenance treatment, with every other day dosing (QOD), was continued for up to 1 year in patients achieving antitumor and antiviral responses. The median time to response was 2 months and the median duration of response was 5 months in the asymptomatic patients.
In all studies, the likelihood of response was greatest in patients with relatively intact immune systems as assessed by baseline CD4 counts (interchangeable with T4 counts). Results at doses of 30 million IU/m2 TIW and 35 million IU/QD were subcutaneously similar and are provided together in TABLE 1. This table demonstrates the relationship of response to baseline CD4 count in both asymptomatic and symptomatic patients in the 30 million IU/m2 TIW and the 35 million IU/QD treatment groups.
In the 30 million IU study group, 7% (5/72) of patients were complete responders and 22% (16/72) of the patients were partial responders. The 35 million IU study had 13% (3/23 patients) complete responders and 17% (4/23) partial responders.
For patients who received 30 million IU TIW, the median survival time was longer in patients with CD4 greater than 200 (30.7 months) than in patients with CD4 less than or equal to 200 (8.9 months). Among responders, the median survival time was 22.6 months versus 9.7 months in nonresponders.
Chronic Hepatitis C
The safety and efficacy of INTRON A in the treatment of chronic hepatitis C was evaluated in 5 randomized clinical studies in which an INTRON A dose of 3 million IU three times a week (TIW) was assessed. The initial three studies were placebo-controlled trials that evaluated a 6-month (24-week) course of therapy. In each of the three studies, INTRON A therapy resulted in a reduction in serum alanine aminotransferase (ALT) in a greater proportion of patients versus control patients at the end of 6 months of dosing. During the 6 months of follow-up, approximately 50% of the patients who responded maintained their ALT response. A combined analysis comparing pretreatment and post-treatment liver biopsies revealed histological improvement in a statistically significantly greater proportion of INTRON A-treated patients compared to controls.
Two additional studies have investigated longer treatment durations (up to 24 months).5,6 Patients in the two studies to evaluate longer duration of treatment had hepatitis with or without cirrhosis in the absence of decompensated liver disease. Complete response to treatment was defined as normalization of the final two serum ALT levels during the treatment period. A sustained response was defined as a complete response at the end of the treatment period, with sustained normal ALT values lasting at least 6 months following discontinuation of therapy.
In Study 1, all patients were initially treated with INTRON A 3 million IU TIW subcutaneously for 24 weeks (run-in-period). Patients who completed the initial 24-week treatment period were then randomly assigned to receive no further treatment, or to receive 3 million IU TIW for an additional 48 weeks. In Study 2, patients who met the entry criteria were randomly assigned to receive INTRON A 3 million IU TIW subcutaneously for 24 weeks or to receive INTRON A 3 million IU TIW subcutaneously for 96 weeks. In both studies, patient follow-up was variable and some data collection was retrospective.
Results show that longer durations of INTRON A therapy improved the sustained response rate (see TABLE 2). In patients with complete responses (CR) to INTRON A therapy after 6 months of treatment (149/352 [42%]), responses were less often sustained if drug was discontinued (21/70 [30%]) than if it was continued for 18 to 24 months (44/79 [56%]). Of all patients randomized, the sustained response rate in the patients receiving 18 or 24 months of therapy was 22% and 26%, respectively, in the two trials. In patients who did not have a CR by 6 months, additional therapy did not result in significantly more responses, since almost all patients who responded to therapy did so within the first 16 weeks of treatment.
A subset (less than 50%) of patients from the combined extended dosing studies had liver biopsies performed both before and after INTRON A treatment. Improvement in necroinflammatory activity as assessed retrospectively by the Knodell (Study 1) and Scheuer (Study 2) Histology Activity Indices was observed in both studies. A higher number of patients (58%, 45/78) improved with extended therapy than with shorter (6 months) therapy (38%, 34/89) in this subset.
Combination treatment with INTRON A and REBETOL® (ribavirin USP) provided a significant reduction in virologic load and improved histologic response in adult patients with compensated liver disease who were treatment-naïve or had relapsed following therapy with alpha interferon alone; pediatric patients previously untreated with alpha interferon experienced a sustained virologic response. See REBETOL prescribing information for additional information.
Chronic Hepatitis B
Adults
The safety and efficacy of INTRON A in the treatment of chronic hepatitis B were evaluated in three clinical trials in which INTRON A doses of 30 to 35 million IU per week were administered subcutaneously (SC), as either 5 million IU daily (QD), or 10 million IU three times a week (TIW) for 16 weeks versus no treatment. All patients were 18 years of age or older with compensated liver disease, and had chronic hepatitis B virus (HBV) infection (serum HBsAg positive for at least 6 months) and HBV replication (serum HBeAg positive). Patients were also serum HBV-DNA positive, an additional indicator of HBV replication, as measured by a research assay.7,8 All patients had elevated serum alanine aminotransferase (ALT) and liver biopsy findings compatible with the diagnosis of chronic hepatitis. Patients with the presence of antibody to human immunodeficiency virus (anti-HIV) or antibody to hepatitis delta virus (anti-HDV) in the serum were excluded from the studies.
Virologic response to treatment was defined in these studies as a loss of serum markers of HBV replication (HBeAg and HBV DNA). Secondary parameters of response included loss of serum HBsAg, decreases in serum ALT, and improvement in liver histology.
In each of two randomized controlled studies, a significantly greater proportion of INTRON A-treated patients exhibited a virologic response compared with untreated control patients (see TABLE 3). In a third study without a concurrent control group, a similar response rate to INTRON A therapy was observed. Pretreatment with prednisone, evaluated in two of the studies, did not improve the response rate and provided no additional benefit.
The response to INTRON A therapy was durable. No patient responding to INTRON A therapy at a dose of 5 million IU QD or 10 million IU TIW relapsed during the follow-up period, which ranged from 2 to 6 months after treatment ended. The loss of serum HBeAg and HBV DNA was maintained in 100% of 19 responding patients followed for 3.5 to 36 months after the end of therapy.
In a proportion of responding patients, loss of HBeAg was followed by the loss of HBsAg. HBsAg was lost in 27% (4/15) of patients who responded to INTRON A therapy at a dose of 5 million IU QD, and 35% (8/23) of patients who responded to 10 million IU TIW. No untreated control patient lost HBsAg in these studies.
In an ongoing study to assess the long-term durability of virologic response, 64 patients responding to INTRON A therapy have been followed for 1.1 to 6.6 years after treatment; 95% (61/64) remain serum HBeAg negative, and 49% (30/61) lost serum HBsAg.
INTRON A therapy resulted in normalization of serum ALT in a significantly greater proportion of treated patients compared to untreated patients in each of two controlled studies (see TABLE 4). In a third study without a concurrent control group, normalization of serum ALT was observed in 50% (12/24) of patients receiving INTRON A therapy.
Virologic response was associated with a reduction in serum ALT to normal or near normal (less than or equal to 1.5 × the upper limit of normal) in 87% (13/15) of patients responding to INTRON A therapy at 5 million IU QD, and 100% (23/23) of patients responding to 10 million IU TIW.
Improvement in liver histology was evaluated in Studies 1 and 3 by comparison of pretreatment and 6-month post-treatment liver biopsies using the semiquantitative Knodell Histology Activity Index.9 No statistically significant difference in liver histology was observed in treated patients compared to control patients in Study 1. Although statistically significant histological improvement from baseline was observed in treated patients in Study 3 (P≤0.01), there was no control group for comparison. Of those patients exhibiting a virologic response following treatment with 5 million IU QD or 10 million IU TIW, histological improvement was observed in 85% (17/20) compared to 36% (9/25) of patients who were not virologic responders. The histological improvement was due primarily to decreases in severity of necrosis, degeneration, and inflammation in the periportal, lobular, and portal regions of the liver (Knodell Categories I + II + III). Continued histological improvement was observed in four responding patients who lost serum HBsAg and were followed 2 to 4 years after the end of INTRON A therapy.10
Pediatrics
The safety and efficacy of INTRON A in the treatment of chronic hepatitis B was evaluated in one randomized controlled trial of 149 patients ranging from 1 year to 17 years of age. Seventy-two patients were treated with 3 million IU/m2 of INTRON A therapy administered subcutaneously three times a week (TIW) for 1 week; the dose was then escalated to 6 million IU/m2 TIW for a minimum of 16 weeks up to 24 weeks. The maximum weekly dosage was 10 million IU TIW. Seventy-seven patients were untreated controls. Study entry and response criteria were identical to those described in the adult patient population.
Patients treated with INTRON A therapy had a better response (loss of HBV DNA and HBeAg at 24 weeks of follow-up) compared to the untreated controls (24% [17/72] versus 10% [8/77] P=0.05). Sixteen of the 17 responders treated with INTRON A therapy remained HBV DNA and HBeAg negative and had a normal serum ALT 12 to 24 months after completion of treatment. Serum HBsAg became negative in 7 out of 17 patients who responded to INTRON A therapy. None of the control patients who had an HBV DNA and HBeAg response became HBsAg negative. At 24 weeks of follow-up, normalization of serum ALT was similar in patients treated with INTRON A therapy (17%, 12/72) and in untreated control patients (16%, 12/77). Patients with a baseline HBV DNA less than 100 pg/mL were more likely to respond to INTRON A therapy than were patients with a baseline HBV DNA greater than 100 pg/mL (35% versus 9%, respectively). Patients who contracted hepatitis B through maternal vertical transmission had lower response rates than those who contracted the disease by other means (5% versus 31%, respectively). There was no evidence that the effects on HBV DNA and HBeAg were limited to specific subpopulations based on age, gender, or race.
TABLE 1 RESPONSE BY BASELINE CD4 COUNT* IN AIDS-RELATED KS PATIENTS 30 million IU/m2 TIW, SC and 35 million IU QD, SC Asymptomatic Symptomatic - * Data for CD4, and asymptomatic and symptomatic classification were not available for all patients.
CD4<200 4/14 (29%) 0/19 (0%) 200≤CD4≤400 6/12 (50%) 0/5 (0%) } 58% CD4>400 5/7 (71%) 0/0 (0%) TABLE 2 SUSTAINED ALT RESPONSE RATE VERSUS DURATION OF THERAPY IN CHRONIC HEPATITIS C PATIENTS INTRON A 3 Million IU TIW Treatment Group* - Number of Patients (%) Study Number INTRON A 3 million IU 24 weeks of treatment INTRON A 3 million IU 72 or 96 weeks of treatment† Difference (Extended — 24 weeks)
(95% CI)‡- * Intent-to-treat groups.
- † Study 1: 72 weeks of treatment; Study 2: 96 weeks of treatment.
- ‡ Confidence intervals adjusted for multiple comparisons due to 3 treatment arms in the study.
ALT response at the end of follow-up 1 12/101 (12%) 23/104 (22%) 10% (-3, 24) 2 9/67 (13%) 21/80 (26%) 13% (-4, 30) Combined Studies 21/168 (12.5%) 44/184 (24%) 11.4% (2, 21) ALT response at the end of treatment 1 40/101 (40%) 51/104 (49%) -- 2 32/67 (48%) 35/80 (44%) -- TABLE 3 VIROLOGIC RESPONSE* IN CHRONIC HEPATITIS B PATIENTS Treatment Group† - Number of Patients (%) Study Number INTRON A 5 million IU QD INTRON A 10 million IU TIW Untreated Controls P‡ Value - * Loss of HBeAg and HBV DNA by 6 months post-therapy.
- † Patients pretreated with prednisone not shown.
- ‡ INTRON A treatment group versus untreated control.
- § Untreated control patients evaluated after 24-week observation period. A subgroup subsequently received INTRON A therapy. A direct comparison is not applicable (NA).
17 15/38 (39%) -- -- 3/42 (7%) 0.0009 2 -- -- 10/24 (42%) 1/22 (5%) 0.005 38 -- -- 13/24§ (54%) 2/27 (7%)§ NA§ All Studies 15/38 (39%) 23/48 (48%) 6/91 (7%) -- TABLE 4 ALT RESPONSES* IN CHRONIC HEPATITIS B PATIENTS Treatment Group - Number of Patients (%) Study Number INTRON A 5 million IU QD INTRON A 10 million IU TIW Untreated Controls P† Value - * Reduction in serum ALT to normal by 6 months post-therapy.
- † INTRON A treatment group versus untreated control.
- ‡ Untreated control patients evaluated after 24-week observation period. A subgroup subsequently received INTRON A therapy. A direct comparison is not applicable (NA).
1 16/38 (42%) -- -- 8/42 (19%) 0.03 2 -- -- 10/24 (42%) 1/22 (5%) 0.0034 3 -- -- 12/24‡ (50%) 2/27 (7%)‡ NA‡ All Studies 16/38 (42%) 22/48 (46%) 11/91 (12%) -- -
INDICATIONS AND USAGE
Hairy Cell Leukemia
INTRON® A is indicated for the treatment of patients 18 years of age or older with hairy cell leukemia.
Malignant Melanoma
INTRON A is indicated as adjuvant to surgical treatment in patients 18 years of age or older with malignant melanoma who are free of disease but at high risk for systemic recurrence, within 56 days of surgery.
Follicular Lymphoma
INTRON A is indicated for the initial treatment of clinically aggressive (see Clinical Pharmacology) follicular Non-Hodgkin's Lymphoma in conjunction with anthracycline-containing combination chemotherapy in patients 18 years of age or older. Efficacy of INTRON A therapy in patients with low-grade, low-tumor burden follicular Non-Hodgkin's Lymphoma has not been demonstrated.
Condylomata Acuminata
INTRON A is indicated for intralesional treatment of selected patients 18 years of age or older with condylomata acuminata involving external surfaces of the genital and perianal areas (see DOSAGE AND ADMINISTRATION).
The use of this product in adolescents has not been studied.
AIDS-Related Kaposi's Sarcoma
INTRON A is indicated for the treatment of selected patients 18 years of age or older with AIDS-Related Kaposi's Sarcoma. The likelihood of response to INTRON A therapy is greater in patients who are without systemic symptoms, who have limited lymphadenopathy and who have a relatively intact immune system as indicated by total CD4 count.
Chronic Hepatitis C
INTRON A is indicated for the treatment of chronic hepatitis C in patients 18 years of age or older with compensated liver disease who have a history of blood or blood-product exposure and/or are HCV antibody positive. Studies in these patients demonstrated that INTRON A therapy can produce clinically meaningful effects on this disease, manifested by normalization of serum alanine aminotransferase (ALT) and reduction in liver necrosis and degeneration.
A liver biopsy should be performed to establish the diagnosis of chronic hepatitis. Patients should be tested for the presence of antibody to HCV. Patients with other causes of chronic hepatitis, including autoimmune hepatitis, should be excluded. Prior to initiation of INTRON A therapy, the physician should establish that the patient has compensated liver disease. The following patient entrance criteria for compensated liver disease were used in the clinical studies and should be considered before INTRON A treatment of patients with chronic hepatitis C:
- No history of hepatic encephalopathy, variceal bleeding, ascites, or other clinical signs of decompensation
- Bilirubin
Less than or equal to 2 mg/dL - Albumin
Stable and within normal limits - Prothrombin Time
Less than 3 seconds prolonged - WBC
Greater than or equal to 3000/mm3 - Platelets
Greater than or equal to 70,000/mm3 Serum creatinine should be normal or near normal.
Prior to initiation of INTRON A therapy, CBC and platelet counts should be evaluated in order to establish baselines for monitoring potential toxicity. These tests should be repeated at Weeks 1 and 2 following initiation of INTRON A therapy, and monthly thereafter. Serum ALT should be evaluated at approximately 3-month intervals to assess response to treatment (see DOSAGE AND ADMINISTRATION).
Patients with preexisting thyroid abnormalities may be treated if thyroid-stimulating hormone (TSH) levels can be maintained in the normal range by medication. TSH levels must be within normal limits upon initiation of INTRON A treatment and TSH testing should be repeated at 3 and 6 months (see PRECAUTIONS, Laboratory Tests).
INTRON A in combination with REBETOL® is indicated for the treatment of chronic hepatitis C in patients 3 years of age and older with compensated liver disease previously untreated with alpha interferon therapy and in patients 18 years of age and older who have relapsed following alpha interferon therapy. See REBETOL prescribing information for additional information.
Chronic Hepatitis B
INTRON A is indicated for the treatment of chronic hepatitis B in patients 1 year of age or older with compensated liver disease. Patients who have been serum HBsAg positive for at least 6 months and have evidence of HBV replication (serum HBeAg positive) with elevated serum ALT are candidates for treatment. Studies in these patients demonstrated that INTRON A therapy can produce virologic remission of this disease (loss of serum HBeAg) and normalization of serum aminotransferases. INTRON A therapy resulted in the loss of serum HBsAg in some responding patients.
Prior to initiation of INTRON A therapy, it is recommended that a liver biopsy be performed to establish the presence of chronic hepatitis and the extent of liver damage. The physician should establish that the patient has compensated liver disease. The following patient entrance criteria for compensated liver disease were used in the clinical studies and should be considered before INTRON A treatment of patients with chronic hepatitis B:
- No history of hepatic encephalopathy, variceal bleeding, ascites, or other signs of clinical decompensation
- Bilirubin
Normal - Albumin
Stable and within normal limits - Prothrombin Time
Adults less than 3 seconds prolonged
Pediatrics less than or equal to 2 seconds prolonged- WBC
Greater than or equal to 4000/mm3 - Platelets
Adults greater than or equal to 100,000/mm3
Pediatrics greater than or equal to 150,000/mm3Patients with causes of chronic hepatitis other than chronic hepatitis B or chronic hepatitis C should not be treated with INTRON A. CBC and platelet counts should be evaluated prior to initiation of INTRON A therapy in order to establish baselines for monitoring potential toxicity. These tests should be repeated at treatment Weeks 1, 2, 4, 8, 12, and 16. Liver function tests, including serum ALT, albumin, and bilirubin, should be evaluated at treatment Weeks 1, 2, 4, 8, 12, and 16. HBeAg, HBsAg, and ALT should be evaluated at the end of therapy, as well as 3- and 6-months post-therapy, since patients may become virologic responders during the 6-month period following the end of treatment. In clinical studies in adults, 39% (15/38) of responding patients lost HBeAg 1 to 6 months following the end of INTRON A therapy. Of responding patients who lost HBsAg, 58% (7/12) did so 1 to 6 months post-treatment.
A transient increase in ALT greater than or equal to 2 times baseline value (flare) can occur during INTRON A therapy for chronic hepatitis B. In clinical trials in adults and pediatrics, this flare generally occurred 8 to 12 weeks after initiation of therapy and was more frequent in responders (adults 63%, 24/38; pediatrics 59%, 10/17) than in nonresponders (adults 27%, 13/48; pediatrics 35%, 19/55). However, in adults and pediatrics, elevations in bilirubin greater than or equal to 3 mg/dL (greater than or equal to 2 times ULN) occurred infrequently (adults 2%, 2/86; pediatrics 3%, 2/72) during therapy. When ALT flare occurs, in general, INTRON A therapy should be continued unless signs and symptoms of liver failure are observed. During ALT flare, clinical symptomatology and liver function tests including ALT, prothrombin time, alkaline phosphatase, albumin, and bilirubin, should be monitored at approximately 2-week intervals (see WARNINGS).
-
CONTRAINDICATIONS
INTRON® A is contraindicated in patients with:
- Hypersensitivity to interferon alpha or any component of the product
- Autoimmune hepatitis
- Decompensated liver disease
INTRON A and REBETOL® combination therapy is additionally contraindicated in:
- Patients with hypersensitivity to ribavirin or any other component of the product
- Women who are pregnant
- Men whose female partners are pregnant
- Patients with hemoglobinopathies (e.g., thalassemia major, sickle cell anemia)
- Patients with creatinine clearance less than 50 mL/min.
See REBETOL prescribing information for additional information.
-
WARNINGS
General
Moderate to severe adverse experiences may require modification of the patient's dosage regimen, or in some cases termination of INTRON® A therapy. Because of the fever and other "flu-like" symptoms associated with INTRON A administration, it should be used cautiously in patients with debilitating medical conditions, such as those with a history of pulmonary disease (e.g., chronic obstructive pulmonary disease) or diabetes mellitus prone to ketoacidosis. Caution should also be observed in patients with coagulation disorders (e.g., thrombophlebitis, pulmonary embolism) or severe myelosuppression.
Cardiovascular Disorders
INTRON A therapy should be used cautiously in patients with a history of cardiovascular disease. Those patients with a history of myocardial infarction and/or previous or current arrhythmic disorder who require INTRON A therapy should be closely monitored (see PRECAUTIONS, Laboratory Tests). Cardiovascular adverse experiences, which include hypotension, arrhythmia, or tachycardia of 150 beats per minute or greater, and rarely, cardiomyopathy and myocardial infarction have been observed in some INTRON A-treated patients. Some patients with these adverse events had no history of cardiovascular disease. Transient cardiomyopathy was reported in approximately 2% of the AIDS-Related Kaposi's Sarcoma patients treated with INTRON A. Hypotension may occur during INTRON A administration, or up to 2 days post-therapy, and may require supportive therapy including fluid replacement to maintain intravascular volume.
Supraventricular arrhythmias occurred rarely and appeared to be correlated with preexisting conditions and prior therapy with cardiotoxic agents. These adverse experiences were controlled by modifying the dose or discontinuing treatment, but may require specific additional therapy.
Cerebrovascular Disorders
Ischemic and hemorrhagic cerebrovascular events have been observed in patients treated with interferon alpha-based therapies, including INTRON A. Events occurred in patients with few or no reported risk factors for stroke, including patients less than 45 years of age. Because these are spontaneous reports, estimates of frequency cannot be made and a causal relationship between interferon alpha-based therapies and these events is difficult to establish.
Neuropsychiatric Disorders
DEPRESSION AND SUICIDAL BEHAVIOR INCLUDING SUICIDAL IDEATION, SUICIDAL ATTEMPTS, AND COMPLETED SUICIDES, HOMICIDAL IDEATION, AND AGGRESSIVE BEHAVIOR SOMETIMES DIRECTED TOWARDS OTHERS, HAVE BEEN REPORTED IN ASSOCIATION WITH TREATMENT WITH ALPHA INTERFERONS, INCLUDING INTRON A THERAPY. If patients develop psychiatric problems, including clinical depression, it is recommended that the patients be carefully monitored during treatment and in the 6-month follow-up period.
INTRON A should be used with caution in patients with a history of psychiatric disorders. INTRON A therapy should be discontinued for any patient developing severe psychiatric disorder during treatment. Obtundation and coma have also been observed in some patients, usually elderly, treated at higher doses. While these effects are usually rapidly reversible upon discontinuation of therapy, full resolution of symptoms has taken up to 3 weeks in a few severe episodes. If psychiatric symptoms persist or worsen, or suicidal or homicidal ideation or aggressive behavior towards others is identified, discontinue treatment with INTRON A and follow the patient closely, with psychiatric intervention as appropriate. Narcotics, hypnotics, or sedatives may be used concurrently with caution and patients should be closely monitored until the adverse effects have resolved. Suicidal ideation or attempts occurred more frequently among pediatric patients, primarily adolescents, compared to adult patients (2.4% versus 1%) during treatment and off-therapy follow-up. Cases of encephalopathy have also been observed in some patients, usually elderly, treated with higher doses of INTRON A.
Treatment with interferons may be associated with exacerbated symptoms of psychiatric disorders in patients with co-occurring psychiatric and substance use disorders. If treatment with interferons is initiated in patients with prior history or existence of psychiatric condition or with a history of substance use disorders, treatment considerations should include the need for drug screening and periodic health evaluation, including psychiatric symptom monitoring. Early intervention for re-emergence or development of neuropsychiatric symptoms and substance use is recommended.
Bone Marrow Toxicity
INTRON A therapy suppresses bone marrow function and may result in severe cytopenias including aplastic anemia. It is advised that complete blood counts (CBC) be obtained pretreatment and monitored routinely during therapy (see PRECAUTIONS, Laboratory Tests). INTRON A therapy should be discontinued in patients who develop severe decreases in neutrophil (less than 0.5 × 109/L) or platelet counts (less than 25 × 109/L) (see DOSAGE AND ADMINISTRATION, Guidelines for Dose Modification).
Ophthalmologic Disorders
Decrease or loss of vision, retinopathy including macular edema, retinal artery or vein thrombosis, retinal hemorrhages and cotton wool spots; optic neuritis, papilledema, and serous retinal detachment may be induced or aggravated by treatment with interferon alfa-2b or other alpha interferons. All patients should receive an eye examination at baseline. Patients with preexisting ophthalmologic disorders (e.g., diabetic or hypertensive retinopathy) should receive periodic ophthalmologic exams during interferon alpha treatment. Any patient who develops ocular symptoms should receive a prompt and complete eye examination. Interferon alfa-2b treatment should be discontinued in patients who develop new or worsening ophthalmologic disorders.
Endocrine Disorders
Infrequently, patients receiving INTRON A therapy developed thyroid abnormalities, either hypothyroid or hyperthyroid. The mechanism by which INTRON A may alter thyroid status is unknown. Patients with preexisting thyroid abnormalities whose thyroid function cannot be maintained in the normal range by medication should not be treated with INTRON A. Prior to initiation of INTRON A therapy, serum TSH should be evaluated. Patients developing symptoms consistent with possible thyroid dysfunction during the course of INTRON A therapy should have their thyroid function evaluated and appropriate treatment instituted. Therapy should be discontinued for patients developing thyroid abnormalities during treatment whose thyroid function cannot be normalized by medication. Discontinuation of INTRON A therapy has not always reversed thyroid dysfunction occurring during treatment. Diabetes mellitus has been observed in patients treated with alpha interferons. Patients with these conditions who cannot be effectively treated by medication should not begin INTRON A therapy. Patients who develop these conditions during treatment and cannot be controlled with medication should not continue INTRON A therapy.
Gastrointestinal Disorders
Hepatotoxicity, including fatality, has been observed in interferon alpha-treated patients, including those treated with INTRON A. INTRON A increases the risk of hepatic decompensation and death in patients with cirrhosis. Any patient developing liver function abnormalities during treatment should be monitored closely and if appropriate, treatment should be discontinued.
Pulmonary Disorders
Dyspnea, pulmonary infiltrates, pneumonia, bronchiolitis obliterans, interstitial pneumonitis, pulmonary hypertension, and sarcoidosis, some resulting in respiratory failure and/or patient deaths, may be induced or aggravated by INTRON A or other alpha interferons. Recurrence of respiratory failure has been observed with interferon rechallenge. The etiologic explanation for these pulmonary findings has yet to be established. Any patient developing fever, cough, dyspnea, or other respiratory symptoms should have a chest X-ray taken. If the chest X-ray shows pulmonary infiltrates or there is evidence of pulmonary function impairment, the patient should be closely monitored, and, if appropriate, interferon alpha treatment should be discontinued. While this has been reported more often in patients with chronic hepatitis C treated with interferon alpha, it has also been reported in patients with oncologic diseases treated with interferon alpha.
Autoimmune Disorders
Rare cases of autoimmune diseases including thrombocytopenia, vasculitis, Raynaud's phenomenon, rheumatoid arthritis, lupus erythematosus, and rhabdomyolysis have been observed in patients treated with alpha interferons, including patients treated with INTRON A. In very rare cases the event resulted in fatality. The mechanism by which these events developed and their relationship to interferon alpha therapy is not clear. Any patient developing an autoimmune disorder during treatment should be closely monitored and, if appropriate, treatment should be discontinued.
Human Albumin
The powder formulations of this product contain albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin.
AIDS-Related Kaposi's Sarcoma
INTRON A therapy should not be used for patients with rapidly progressive visceral disease (see CLINICAL PHARMACOLOGY). Also of note, there may be synergistic adverse effects between INTRON A and zidovudine. Patients receiving concomitant zidovudine have had a higher incidence of neutropenia than that expected with zidovudine alone. Careful monitoring of the WBC count is indicated in all patients who are myelosuppressed and in all patients receiving other myelosuppressive medications. The effects of INTRON A when combined with other drugs used in the treatment of AIDS-related disease are unknown.
Chronic Hepatitis C and Chronic Hepatitis B
Patients with decompensated liver disease, autoimmune hepatitis or a history of autoimmune disease, and patients who are immunosuppressed transplant recipients should not be treated with INTRON A. There are reports of worsening liver disease, including jaundice, hepatic encephalopathy, hepatic failure, and death following INTRON A therapy in such patients. Therapy should be discontinued for any patient developing signs and symptoms of liver failure.
Chronic hepatitis B patients with evidence of decreasing hepatic synthetic functions, such as decreasing albumin levels or prolongation of prothrombin time, who nevertheless meet the entry criteria to start therapy, may be at increased risk of clinical decompensation if a flare of aminotransferases occurs during INTRON A treatment. In such patients, if increases in ALT occur during INTRON A therapy for chronic hepatitis B, they should be followed carefully, including close monitoring of clinical symptomatology and liver function tests including ALT, prothrombin time, alkaline phosphatase, albumin, and bilirubin. In considering these patients for INTRON A therapy, the potential risks must be evaluated against the potential benefits of treatment.
Peripheral Neuropathy
Peripheral neuropathy has been reported when alpha interferons were given in combination with telbivudine. In one clinical trial, an increased risk and severity of peripheral neuropathy was observed with the combination use of telbivudine and pegylated interferon alfa-2a as compared to telbivudine alone. The safety and efficacy of telbivudine in combination with interferons for the treatment of chronic hepatitis B has not been demonstrated.
Use with Ribavirin (see also REBETOL® prescribing information)
REBETOL may cause birth defects and/or death of the unborn child. REBETOL therapy should not be started until a report of a negative pregnancy test has been obtained immediately prior to planned initiation of therapy. Patients should use at least two forms of contraception and have monthly pregnancy tests (see CONTRAINDICATIONS and PRECAUTIONS, Information for Patients).
Combination treatment with INTRON A and REBETOL was associated with hemolytic anemia. Hemoglobin less than 10 g/dL was observed in approximately 10% of adult and pediatric patients in clinical trials. Anemia occurred within 1 to 2 weeks of initiation of ribavirin therapy. Combination treatment with INTRON A and REBETOL should not be used in patients with creatinine clearance less than 50 mL/min. See REBETOL prescribing information for additional information.
-
PRECAUTIONS
General
Acute serious hypersensitivity reactions (e.g., urticaria, angioedema, bronchoconstriction, anaphylaxis) have been observed rarely in INTRON® A-treated patients; if such an acute reaction develops, the drug should be discontinued immediately and appropriate medical therapy instituted. Transient rashes have occurred in some patients following injection, but have not necessitated treatment interruption.
While fever may be related to the flu-like syndrome reported commonly in patients treated with interferon, other causes of persistent fever should be ruled out.
There have been reports of interferon, including INTRON A, exacerbating preexisting psoriasis and sarcoidosis as well as development of new sarcoidosis. Therefore, INTRON A therapy should be used in these patients only if the potential benefit justifies the potential risk.
Variations in dosage, routes of administration, and adverse reactions exist among different brands of interferon. Therefore, do not use different brands of interferon in any single treatment regimen.
Triglycerides
Elevated triglyceride levels have been observed in patients treated with interferons, including INTRON A therapy. Elevated triglyceride levels should be managed as clinically appropriate. Hypertriglyceridemia may result in pancreatitis. Discontinuation of INTRON A therapy should be considered for patients with persistently elevated triglycerides (e.g., triglycerides greater than 1000 mg/dL) associated with symptoms of potential pancreatitis, such as abdominal pain, nausea, or vomiting.
Drug Interactions
Interactions between INTRON A and other drugs have not been fully evaluated. Caution should be exercised when administering INTRON A therapy in combination with other potentially myelosuppressive agents such as zidovudine. Concomitant use of alpha interferon and theophylline decreases theophylline clearance, resulting in a 100% increase in serum theophylline levels.
Information for Patients
Patients receiving INTRON A alone or in combination with REBETOL® should be informed of the risks and benefits associated with treatment and should be instructed on proper use of the product. To supplement your discussion with a patient, you may wish to provide patients with a copy of the MEDICATION GUIDE.
Patients should be informed of, and advised to seek medical attention for, symptoms indicative of serious adverse reactions associated with this product. Such adverse reactions may include depression (suicidal ideation), cardiovascular (chest pain), ophthalmologic toxicity (decrease in/or loss of vision), pancreatitis or colitis (severe abdominal pain), and cytopenias (high persistent fevers, bruising, dyspnea). Patients should be advised that some side effects such as fatigue and decreased concentration might interfere with the ability to perform certain tasks. Patients who are taking INTRON A in combination with REBETOL must be thoroughly informed of the risks to a fetus. Female patients and female partners of male patients must be told to use two forms of birth control during treatment and for six months after therapy is discontinued (see MEDICATION GUIDE).
Patients should be advised to remain well hydrated during the initial stages of treatment and that use of an antipyretic may ameliorate some of the flu-like symptoms.
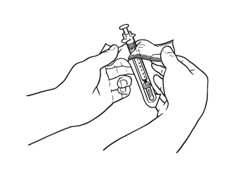
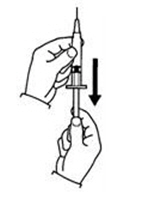
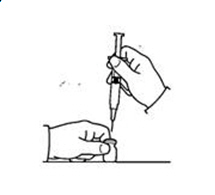
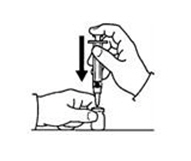
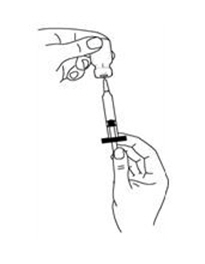
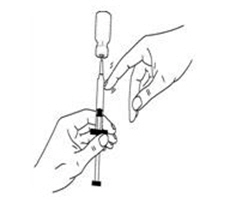
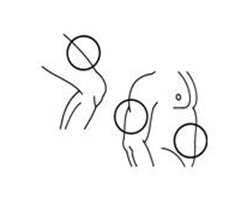
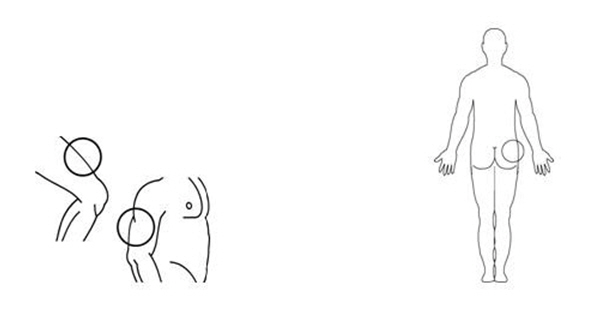
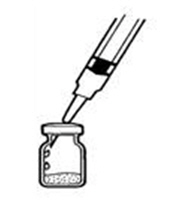
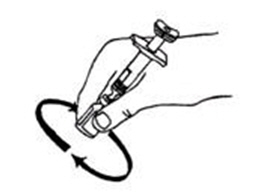
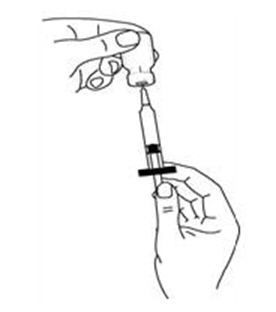
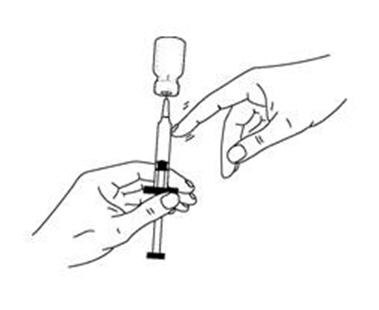
If a decision is made to allow a patient to self-administer INTRON A, they should be instructed, based on their treatment, if they should inject a dose of INTRON® A subcutaneously or intramuscularly. If it is too difficult for them to inject themselves, they should be instructed to ask someone who has been trained to give the injection to them. Patients should be instructed on the importance of site selection for self-administering the injection, as well as the importance on rotating the injection sites. A puncture resistant container for the disposal of needles and syringes should be supplied. Patients self-administering INTRON A should be instructed on the proper disposal of needles and syringes and cautioned against reuse.
Patients should be instructed that the Sterile Water for Injection vial supplied with Intron A Powder for Injection contains an excess amount of diluent (5 mL) and only 1 mL should be withdrawn to reconstitute Intron A Powder for Injection. The vial of Sterile Water for Injection is intended for single-dose only. Discard the unused portion of sterile water. Do not save or reuse.
Dental and Periodontal Disorders
Dental and periodontal disorders have been reported in patients receiving ribavirin and interferon combination therapy. In addition, dry mouth could have a damaging effect on teeth and mucous membranes of the mouth during long-term treatment with the combination of REBETOL and interferon alfa-2b. Patients should brush their teeth thoroughly twice daily and have regular dental examinations. In addition, some patients may experience vomiting. If this reaction occurs, they should be advised to rinse out their mouth thoroughly afterwards.
Laboratory Tests
In addition to those tests normally required for monitoring patients, the following laboratory tests are recommended for all patients on INTRON A therapy, prior to beginning treatment and then periodically thereafter.
- Standard hematologic tests — including hemoglobin, complete and differential white blood cell counts, and platelet count.
- Blood chemistries — electrolytes, liver function tests, and TSH.
- Monitor hepatic function with serum bilirubin, ALT (alanine transaminase), AST (aspartate aminotransferase), alkaline phosphatase, and LDH (lactate dehydrogenase) at 2, 8 and 12 weeks following initiation of INTRON A, then every 6 months while receiving INTRON A. Permanently discontinue INTRON A for evidence of severe (Grade 3) hepatic injury or hepatic decompensation (Child-Pugh score >6 [class B and C]).
Those patients who have preexisting cardiac abnormalities and/or are in advanced stages of cancer should have electrocardiograms taken prior to and during the course of treatment.
Mild-to-moderate leukopenia and elevated serum liver enzyme (SGOT) levels have been reported with intralesional administration of INTRON A (see ADVERSE REACTIONS); therefore, the monitoring of these laboratory parameters should be considered.
Baseline chest X-rays are suggested and should be repeated if clinically indicated.
For malignant melanoma patients, differential WBC count and liver function tests should be monitored weekly during the induction phase of therapy and monthly during the maintenance phase of therapy.
For specific recommendations in chronic hepatitis C and chronic hepatitis B, see INDICATIONS AND USAGE.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with INTRON A have not been performed to determine carcinogenicity.
Interferon may impair fertility. In studies of interferon administration in nonhuman primates, menstrual cycle abnormalities have been observed. Decreases in serum estradiol and progesterone concentrations have been reported in women treated with human leukocyte interferon.12 Therefore, fertile women should not receive INTRON A therapy unless they are using effective contraception during the therapy period. INTRON A therapy should be used with caution in fertile men.
Mutagenicity studies have demonstrated that INTRON A is not mutagenic.
Studies in mice (0.1, 1.0 million IU/day), rats (4, 20, 100 million IU/kg/day), and cynomolgus monkeys (1.1 million IU/kg/day; 0.25, 0.75, 2.5 million IU/kg/day) injected with INTRON A for up to 9 days, 3 months, and 1 month, respectively, have revealed no evidence of toxicity. However, in cynomolgus monkeys (4, 20, 100 million IU/kg/day) injected daily for 3 months with INTRON A, toxicity was observed at the mid and high doses and mortality was observed at the high dose.
However, due to the known species-specificity of interferon, the effects in animals are unlikely to be predictive of those in man.
INTRON A in combination with REBETOL should be used with caution in fertile men. See the REBETOL prescribing information for additional information.
Pregnancy
INTRON A has been shown to have abortifacient effects in Macaca mulatta (rhesus monkeys) at 15 and 30 million IU/kg (estimated human equivalent of 5 and 10 million IU/kg, based on body surface area adjustment for a 60-kg adult). There are no adequate and well-controlled studies in pregnant women. INTRON A therapy should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
See REBETOL prescribing information for additional information. Significant teratogenic and/or embryocidal effects have been demonstrated in all animal species exposed to ribavirin. REBETOL therapy is contraindicated in women who are pregnant and in the male partners of women who are pregnant. See CONTRAINDICATIONS and the REBETOL prescribing information.
Ribavirin Pregnancy Registry
A Ribavirin Pregnancy Registry has been established to monitor maternal-fetal outcomes of pregnancies in female patients and female partners of male patients exposed to ribavirin during treatment and for 6 months following cessation of treatment. Physicians and patients are encouraged to report such cases by calling 1-800-593-2214.
Nursing Mothers
It is not known whether this drug is excreted in human milk. However, studies in mice have shown that mouse interferons are excreted into the milk. Because of the potential for serious adverse reactions from the drug in nursing infants, a decision should be made whether to discontinue nursing or to discontinue INTRON A therapy, taking into account the importance of the drug to the mother.
Pediatric Use
General
Safety and effectiveness in pediatric patients have not been established for indications other than chronic hepatitis B and chronic hepatitis C.
Chronic Hepatitis B
Safety and effectiveness in pediatric patients ranging in age from 1 to 17 years have been established based upon one controlled clinical trial (see CLINICAL PHARMACOLOGY, INDICATIONS AND USAGE, and DOSAGE AND ADMINISTRATION, Chronic Hepatitis B Pediatrics).
Chronic Hepatitis C
Safety and effectiveness in pediatric patients ranging in age from 3 to 16 years have been established based upon clinical studies in 118 patients. See REBETOL prescribing information for additional information. Suicidal ideation or attempts occurred more frequently among pediatric patients compared to adult patients (2.4% versus 1%) during treatment and off-therapy follow-up (see WARNINGS, Neuropsychiatric Disorders). During a 48-week course of therapy there was a decrease in the rate of linear growth (mean percentile assignment decrease of 7%) and a decrease in the rate of weight gain (mean percentile assignment decrease of 9%). A general reversal of these trends was noted during the 24-week post-treatment period.
Long-term data in a limited number of patients suggests that combination therapy may induce a growth inhibition that results in reduced final adult height in some patients (see ADVERSE REACTIONS, Chronic Hepatitis C Pediatrics).
Geriatric Use
In all clinical studies of INTRON A, including studies as monotherapy and in combination with REBETOL (ribavirin USP) Capsules, only a small percentage of the subjects were aged 65 and over. These numbers were too few to determine if they respond differently from younger subjects except for the clinical trials of INTRON A in combination with REBETOL, where elderly subjects had a higher frequency of anemia (67%) than did younger patients (28%).
In a database consisting of clinical study and postmarketing reports for various indications, cardiovascular adverse events and confusion were reported more frequently in elderly patients receiving INTRON A therapy compared to younger patients.
In general, INTRON A therapy should be administered to elderly patients cautiously, reflecting the greater frequency of decreased hepatic, renal, bone marrow, and/or cardiac function and concomitant disease or other drug therapy. INTRON A is known to be substantially excreted by the kidney, and the risk of adverse reactions to INTRON A may be greater in patients with impaired renal function. Because elderly patients often have decreased renal function, patients should be carefully monitored during treatment, and dose adjustments made based on symptoms and/or laboratory abnormalities (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
General
The adverse experiences listed below were reported to be possibly or probably related to INTRON® A therapy during clinical trials. Most of these adverse reactions were mild to moderate in severity and were manageable. Some were transient and most diminished with continued therapy.
The most frequently reported adverse reactions were "flu-like" symptoms, particularly fever, headache, chills, myalgia, and fatigue. More severe toxicities are observed generally at higher doses and may be difficult for patients to tolerate.
TREATMENT-RELATED ADVERSE EXPERIENCES BY INDICATION Dosing Regimens
Percentage (%) of Patients*MALIGNANT MELANOMA FOLLICULAR LYMPHOMA HAIRY CELL LEUKEMIA CONDYLOMATA ACUMINATA AIDS-RELATED KAPOSI'S SARCOMA CHRONIC HEPATITIS C† CHRONIC HEPATITIS B Adults Pediatrics 20 MIU/m2
Induction (IV)
10 MIU/m2
Maintenance
(SC)5 MIU
TIW/SC
2 MIU/m2
TIW/SC
1 MIU/lesion 30 MIU/m2
TIW/SC
35 MIU
QD/SC
3 MIU
TIW
5 MIU
QD
10 MIU
TIW
6 MIU/m2
TIW
ADVERSE EXPERIENCE N=143 N=135 N=145 N=352 N=74 N=29 N=183 N=101 N=78 N=116 - * Dash (--) indicates not reported
- † Percentages based upon a summary of all adverse events during 18 to 24 months of treatment
- ‡ Vomiting was reported with nausea as a single term
- § Includes stomatitis/mucositis
- ¶ Amnesia was reported with confusion as a single term
- # Predominantly lethargy
Application-Site Disorders 20 injection site
inflammation-- 1 -- -- -- -- 5 3 -- -- other (≤5%) burning, injection site bleeding, injection site pain, injection site reaction (5% in chronic hepatitis B pediatrics), itching Blood Disorders (<5%) anemia, anemia hypochromic, granulocytopenia, hemolytic anemia, leukopenia, lymphocytosis, neutropenia (9% in chronic hepatitis C, 14% in chronic hepatitis B pediatrics), thrombocytopenia (10% in chronic hepatitis C) (bleeding 8% in malignant melanoma), thrombocytopenia purpura Body as a Whole facial edema -- 1 -- <1 -- 10 <1 3 1 <1 weight decrease 3 13 <1 <1 5 3 10 2 5 3 other (≤5%) allergic reaction, cachexia, dehydration, earache, hernia, edema, hypercalcemia, hyperglycemia, hypothermia, inflammation nonspecific, lymphadenitis, lymphadenopathy, mastitis, periorbital edema, poor peripheral circulation, peripheral edema (6% in follicular lymphoma), phlebitis superficial, scrotal/penile edema, thirst, weakness, weight increase Cardiovascular System Disorders (<5%) angina, arrhythmia, atrial fibrillation, bradycardia, cardiac failure, cardiomegaly, cardiomyopathy, coronary artery disorder, extrasystoles, heart valve disorder, hematoma, hypertension (9% in chronic hepatitis C), hypotension, palpitations, phlebitis, postural hypotension, pulmonary embolism, Raynaud's disease, tachycardia, thrombosis, varicose vein Endocrine System Disorders (<5%) aggravation of diabetes mellitus, goiter, gynecomastia, hyperglycemia, hyperthyroidism, hypertriglyceridemia, hypothyroidism, virilism Flu-like Symptoms fever 81 56 68 56 47 55 34 66 86 94 headache 62 21 39 47 36 21 43 61 44 57 chills 54 -- 46 45 -- -- -- -- -- -- myalgia 75 16 39 44 34 28 43 59 40 27 fatigue 96 8 61 18 84 48 23 75 69 71 increased sweating 6 13 8 2 4 21 4 1 1 3 asthenia -- 63 7 -- 11 -- 40 5 15 5 rigors 2 7 -- -- 30 14 16 38 42 30 arthralgia 6 8 8 9 -- 3 16 19 8 15 dizziness 23 -- 12 9 7 24 9 13 10 8 influenza-like symptoms 10 18 37 -- 45 79 26 5 -- <1 back pain -- 15 19 6 1 3 -- -- -- -- dry mouth 1 2 19 -- 22 28 5 6 5 -- chest pain 2 8 <1 <1 1 28 4 4 -- -- malaise 6 -- -- 14 5 -- 13 9 6 3 pain (unspecified) 15 9 18 3 3 3 -- -- -- -- other (<5%) chest pain substernal, hyperthermia, rhinitis, rhinorrhea Gastrointestinal System Disorders diarrhea 35 19 18 2 18 45 13 19 8 12 anorexia 69 21 19 1 38 41 14 43 53 43 nausea 66 24 21 17 28 21 19 50 33 18 taste alteration 24 2 13 <1 5 7 2 10 -- -- abdominal pain 2 20 <5 1 5 21 16 5 4 23 loose stools -- 1 -- <1 -- 10 2 2 -- 2 vomiting ‡ 32 6 2 11 14 8 7 10 27 constipation 1 14 <1 -- 1 10 4 5 -- 2 gingivitis 2§ 7§ -- -- -- 14 -- 1 -- -- dyspepsia -- 2 -- 2 4 -- 7 3 8 3 other (<5%) abdominal ascites, abdominal distension, colitis, dysphagia, eructation, esophagitis, flatulence, gallstones, gastric ulcer, gastritis, gastroenteritis, gastrointestinal disorder (7% in follicular lymphoma), gastrointestinal hemorrhage, gastrointestinal mucosal discoloration, gingival bleeding, gum hyperplasia, halitosis, hemorrhoids, increased appetite, increased saliva, intestinal disorder, melena, mouth ulceration, mucositis, oral hemorrhage, oral leukoplakia, rectal bleeding after stool, rectal hemorrhage, stomatitis, stomatitis ulcerative, taste loss, tongue disorder, tooth disorder Liver and Biliary System Disorders (<5%) abnormal hepatic function tests, biliary pain, bilirubinemia, hepatitis, increased lactate dehydrogenase, increased transaminases (SGOT/SGPT) (elevated SGOT 63% in malignant melanoma and 24% in follicular lymphoma), jaundice, right upper quadrant pain (15% in chronic hepatitis C), and very rarely, hepatic encephalopathy, hepatic failure, and death Musculoskeletal System Disorders musculoskeletal pain -- 18 -- -- -- -- 21 9 1 10 other (<5%) arteritis, arthritis, arthritis aggravated, arthrosis, bone disorder, bone pain, carpal tunnel syndrome, hyporeflexia, leg cramps, muscle atrophy, muscle weakness, polyarteritis nodosa, tendinitis, rheumatoid arthritis, spondylitis Nervous System and Psychiatric Disorders depression 40 9 6 3 9 28 19 17 6 4 paresthesia 13 13 6 1 3 21 5 6 3 <1 impaired concentration -- 1 -- <1 3 14 3 8 5 3 amnesia ¶ 1 <5 -- -- 14 -- -- -- -- confusion 8 2 <5 4 12 10 1 -- -- 2 hypoesthesia -- 1 <5 1 -- 10 -- -- -- -- irritability 1 1 -- -- -- -- 13 16 12 22 somnolence 1 2 <5 3 3 -- 33# 14 9 5 anxiety 1 9 5 <1 1 3 5 2 -- 3 insomnia 5 4 -- <1 3 3 12 11 6 8 nervousness 1 1 -- 1 -- 3 2 3 -- 3 decreased libido 1 1 <5 -- -- -- 1 5 1 -- other (<5%) abnormal coordination, abnormal dreaming, abnormal gait, abnormal thinking, aggravated depression, aggressive reaction, agitation (7% in chronic hepatitis B pediatrics), alcohol intolerance, apathy, aphasia, ataxia, Bell's palsy, CNS dysfunction, coma, convulsions, delirium, dysphonia, emotional lability, extrapyramidal disorder, feeling of ebriety, flushing, hearing disorder, hearing impairment, hot flashes, hyperesthesia, hyperkinesia, hypertonia, hypokinesia, impaired consciousness, labyrinthine disorder, loss of consciousness, manic depression, manic reaction, migraine, neuralgia, neuritis, neuropathy, neurosis, paresis, paroniria, parosmia, personality disorder, polyneuropathy, psychosis, speech disorder, stroke, suicidal ideation, suicide attempt, syncope, tinnitus, tremor, twitching, vertigo (8% in follicular lymphoma) Reproduction System Disorders (<5%) amenorrhea (12% in follicular lymphoma), dysmenorrhea, impotence, leukorrhea, menorrhagia, menstrual irregularity, pelvic pain, penis disorder, sexual dysfunction, uterine bleeding, vaginal dryness Resistance Mechanism Disorders moniliasis -- 1 -- <1 -- 17 -- -- -- -- herpes simplex 1 2 -- 1 -- 3 1 5 -- -- other (<5%) abscess, conjunctivitis, fungal infection, hemophilus, herpes zoster, infection, infection bacterial, infection nonspecific (7% in follicular lymphoma), infection parasitic, otitis media, sepsis, stye, trichomonas, upper respiratory tract infection, viral infection (7% in chronic hepatitis C) Respiratory System Disorders dyspnea 15 14 <1 -- 1 34 3 5 -- -- coughing 6 13 <1 -- -- 31 1 4 -- 5 pharyngitis 2 8 <5 1 1 31 3 7 1 7 sinusitis 1 4 -- -- -- 21 2 -- -- -- nonproductive coughing 2 7 -- -- -- 14 0 1 -- -- nasal congestion 1 7 -- 1 -- 10 <1 4 -- -- other (≤5%) asthma, bronchitis (10% in follicular lymphoma), bronchospasm, cyanosis, epistaxis (7% in chronic hepatitis B pediatrics), hemoptysis, hypoventilation, laryngitis, lung fibrosis, pleural effusion, orthopnea, pleural pain, pneumonia, pneumonitis, pneumothorax, rales, respiratory disorder, respiratory insufficiency, sneezing, tonsillitis, tracheitis, wheezing Skin and Appendages Disorders dermatitis 1 -- 8 -- -- -- 2 1 -- -- alopecia 29 23 8 -- 12 31 28 26 38 17 pruritus -- 10 11 1 7 -- 9 6 4 3 rash 19 13 25 -- 9 10 5 8 1 5 dry skin 1 3 9 -- 9 10 4 3 -- <1 other (<5%) abnormal hair texture, acne, cellulitis, cyanosis of the hand, cold and clammy skin, dermatitis lichenoides, eczema, epidermal necrolysis, erythema, erythema nodosum, folliculitis, furunculosis, increased hair growth, lacrimal gland disorder, lacrimation, lipoma, maculopapular rash, melanosis, nail disorders, nonherpetic cold sores, pallor, peripheral ischemia, photosensitivity, pruritus genital, psoriasis, psoriasis aggravated, purpura (5% in chronic hepatitis C), rash erythematous, sebaceous cyst, skin depigmentation, skin discoloration, skin nodule, urticaria, vitiligo Urinary System Disorders (<5%) albumin/protein in urine, cystitis, dysuria, hematuria, incontinence, increased BUN, micturition disorder, micturition frequency, nocturia, polyuria (10% in follicular lymphoma), renal insufficiency, urinary tract infection (5% in chronic hepatitis C) Vision Disorders (<5%) abnormal vision, blurred vision, diplopia, dry eyes, eye pain, nystagmus, photophobia Hairy Cell Leukemia
The adverse reactions most frequently reported during clinical trials in 145 patients with hairy cell leukemia were the "flu-like" symptoms of fever (68%), fatigue (61%), and chills (46%).
Malignant Melanoma
The INTRON A dose was modified because of adverse events in 65% (n=93) of the patients. INTRON A therapy was discontinued because of adverse events in 8% of the patients during induction and 18% of the patients during maintenance. The most frequently reported adverse reaction was fatigue, which was observed in 96% of patients. Other adverse reactions that were recorded in greater than 20% of INTRON A-treated patients included neutropenia (92%), fever (81%), myalgia (75%), anorexia (69%), vomiting/nausea (66%), increased SGOT (63%), headache (62%), chills (54%), depression (40%), diarrhea (35%), alopecia (29%), altered taste sensation (24%), dizziness/vertigo (23%), and anemia (22%).
Adverse reactions classified as severe or life threatening (ECOG Toxicity Criteria grade 3 or 4) were recorded in 66% and 14% of INTRON A-treated patients, respectively. Severe adverse reactions recorded in greater than 10% of INTRON A-treated patients included neutropenia/leukopenia (26%), fatigue (23%), fever (18%), myalgia (17%), headache (17%), chills (16%), and increased SGOT (14%). Grade 4 fatigue was recorded in 4% and grade 4 depression was recorded in 2% of INTRON A-treated patients. No other grade 4 AE was reported in more than 2 INTRON A-treated patients. Lethal hepatotoxicity occurred in 2 INTRON A-treated patients early in the clinical trial. No subsequent lethal hepatotoxicities were observed with adequate monitoring of liver function tests (see PRECAUTIONS, Laboratory Tests).
Follicular Lymphoma
Ninety-six percent of patients treated with CHVP plus INTRON A therapy and 91% of patients treated with CHVP alone reported an adverse event of any severity. Asthenia, fever, neutropenia, increased hepatic enzymes, alopecia, headache, anorexia, "flu-like" symptoms, myalgia, dyspnea, thrombocytopenia, paresthesia, and polyuria occurred more frequently in the CHVP plus INTRON A-treated patients than in patients treated with CHVP alone. Adverse reactions classified as severe or life threatening (World Health Organization grade 3 or 4) recorded in greater than 5% of CHVP plus INTRON A-treated patients included neutropenia (34%), asthenia (10%), and vomiting (10%). The incidence of neutropenic infection was 6% in CHVP plus INTRON A versus 2% in CHVP alone. One patient in each treatment group required hospitalization.
Twenty-eight percent of CHVP plus INTRON A-treated patients had a temporary modification/interruption of their INTRON A therapy, but only 13 patients (10%) permanently stopped INTRON A therapy because of toxicity. There were four deaths on study; two patients committed suicide in the CHVP plus INTRON A arm and two patients in the CHVP arm had unwitnessed sudden death. Three patients with hepatitis B (one of whom also had alcoholic cirrhosis) developed hepatotoxicity leading to discontinuation of INTRON A. Other reasons for discontinuation included intolerable asthenia (5/135), severe flu symptoms (2/135), and one patient each with exacerbation of ankylosing spondylitis, psychosis, and decreased ejection fraction.
Condylomata Acuminata
Eighty-eight percent (311/352) of patients treated with INTRON A for condylomata acuminata who were evaluable for safety reported an adverse reaction during treatment. The incidence of the adverse reactions reported increased when the number of treated lesions increased from one to five. All 40 patients who had five warts treated reported some type of adverse reaction during treatment.
Adverse reactions and abnormal laboratory test values reported by patients who were re-treated were qualitatively and quantitatively similar to those reported during the initial INTRON A treatment period.
AIDS-Related Kaposi's Sarcoma
In patients with AIDS-Related Kaposi's Sarcoma, some type of adverse reaction occurred in 100% of the 74 patients treated with 30 million IU/m2 three times a week and in 97% of the 29 patients treated with 35 million IU per day.
Of these adverse reactions, those classified as severe (World Health Organization grade 3 or 4) were reported in 27% to 55% of patients. Severe adverse reactions in the 30 million IU/m2 TIW study included: fatigue (20%), influenza-like symptoms (15%), anorexia (12%), dry mouth (4%), headache (4%), confusion (3%), fever (3%), myalgia (3%), and nausea and vomiting (1% each). Severe adverse reactions for patients who received the 35 million IU QD included: fever (24%), fatigue (17%), influenza-like symptoms (14%), dyspnea (14%), headache (10%), pharyngitis (7%), and ataxia, confusion, dysphagia, GI hemorrhage, abnormal hepatic function, increased SGOT, myalgia, cardiomyopathy, face edema, depression, emotional lability, suicide attempt, chest pain, and coughing (1 patient each). Overall, the incidence of severe toxicity was higher among patients who received the 35 million IU per day dose.
Chronic Hepatitis C
Adults
Two studies of extended treatment (18-24 months) with INTRON A show that approximately 95% of all patients treated experience some type of adverse event and that patients treated for extended duration continue to experience adverse events throughout treatment. Most adverse events reported are mild to moderate in severity. However, 29/152 (19%) of patients treated for 18 to 24 months experienced a serious adverse event compared to 11/163 (7%) of those treated for 6 months. Adverse events which occur or persist during extended treatment are similar in type and severity to those occurring during short-course therapy.
Of the patients achieving a complete response after 6 months of therapy, 12/79 (15%) subsequently discontinued INTRON A treatment during extended therapy because of adverse events, and 23/79 (29%) experienced severe adverse events (WHO grade 3 or 4) during extended therapy.
In patients using combination treatment with INTRON A and REBETOL, the primary toxicity observed was hemolytic anemia. Reductions in hemoglobin levels occurred within the first 1 to 2 weeks of therapy. Cardiac and pulmonary events associated with anemia occurred in approximately 10% of patients treated with INTRON A/REBETOL therapy. See REBETOL prescribing information for additional information.
Chronic Hepatitis C
Pediatrics
In pediatric patients with chronic hepatitis C treated with INTRON A 3 MIU/m2 three times weekly and REBETOL 15 mg/kg per day, all subjects (n=118) had at least one adverse event during 24-48 weeks of treatment, of which 80% were considered to be mild or moderate in severity. Six percent discontinued therapy due to adverse reactions and dose modifications were required in 30% of subjects, most commonly for anemia and neutropenia. Adverse events occurring in more than 50% of subjects included headache, fever, fatigue and anorexia. Adverse events occurring in 20-50% of subjects included influenza-like symptoms, abdominal pain, vomiting, nausea, myalgia, pharyngitis, diarrhea, viral infection, rigors, weight decrease, musculoskeletal pain, alopecia and dizziness. The most common laboratory test abnormalities were neutropenia (34%) and anemia (27%). Depression was reported in 13% (n=15) of children. Three of these subjects had suicidal ideation, and one attempted suicide. Weight loss and slowed growth are common in pediatric patients during combination therapy with INTRON A and REBETOL. Following treatment, rebound growth and weight gain occurred in most subjects. Long-term follow-up data in pediatric subjects, however, indicates that INTRON A in combination with REBETOL may induce a growth inhibition that results in reduced adult height in some patients (see PRECAUTIONS, Pediatric Use).
Chronic Hepatitis B
Adults
In patients with chronic hepatitis B, some type of adverse reaction occurred in 98% of the 101 patients treated at 5 million IU QD and 90% of the 78 patients treated at 10 million IU TIW. Most of these adverse reactions were mild to moderate in severity, were manageable, and were reversible following the end of therapy.
Adverse reactions classified as severe (causing a significant interference with normal daily activities or clinical state) were reported in 21% to 44% of patients. The severe adverse reactions reported most frequently were the "flu-like" symptoms of fever (28%), fatigue (15%), headache (5%), myalgia (4%), rigors (4%), and other severe "flu-like" symptoms, which occurred in 1% to 3% of patients. Other severe adverse reactions occurring in more than one patient were alopecia (8%), anorexia (6%), depression (3%), nausea (3%), and vomiting (2%).
To manage side effects, the dose was reduced, or INTRON A therapy was interrupted in 25% to 38% of patients. Five percent of patients discontinued treatment due to adverse experiences.
Chronic Hepatitis B
Pediatrics
In pediatric patients with chronic hepatitis B (n=72) during 16-24 weeks of treatment, the most frequently reported adverse events were those commonly associated with interferon treatment: flu-like symptoms (100%), gastrointestinal system disorders (46%), and nausea and vomiting (40%). Neutropenia (13%) and thrombocytopenia (3%) were also reported. None of the adverse events was life threatening and most were moderate to severe and resolved upon dose reduction or drug discontinuation.
ABNORMAL LABORATORY TEST VALUES BY INDICATION Dosing Regimens
Percentage (%) of PatientsMALIGNANT MELANOMA FOLLICULAR LYMPHOMA HAIRY CELL LEUKEMIA CONDYLOMATA ACUMINATA AIDS-RELATED KAPOSI'S SARCOMA CHRONIC HEPATITIS C CHRONIC HEPATITIS B Adults Pediatrics 20 MIU/m2
Induction (IV)
10 MIU/m2
Maintenance
(SC)5 MIU
TIW/SC
2 MIU/m2
TIW/SC
1 MIU/lesion 30 MIU/m2
TIW/SC
35 MIU
QD/SC
3 MIU
TIW
5 MIU
QD
10 MIU
TIW
6 MIU/m2
TIW
Laboratory Tests N=143 N=135 N=145 N=352 N=69-73 N=26-28 N=140-171 N=96-101 N=75-103 N=113-115 NA — Not Applicable — Patients' initial hematologic laboratory test values were abnormal due to their condition. - * Decrease of ≥2 g/dL; 20% 2-<3 g/dL; 6% ≥3 g/dL
- † Decrease of ≥2 g/dL
- ‡ Decrease of ≥2 g/dL; 14% 2-<3 g/dL; 3% ≥3 g/dL
- § White Blood Cell Count was reported as neutropenia
- ¶ Decrease to <3000/mm3
- # Decrease to <70,000/mm3
- Þ Neutrophils plus bands
Hemoglobin 22 8 NA -- 1 15 26* 32† 23† 17‡ White Blood Cell Count § -- NA 17 10 22 26¶ 68¶ 34¶ 9¶ Platelet Count 15 13 NA -- 0 8 15# 12# 5# 1# Serum Creatinine 3 2 0 -- -- -- 6 3 0 3 Alkaline Phosphatase 13 -- 4 -- -- -- -- 8 4 0 Lactate Dehydrogenase 1 -- 0 -- -- -- -- -- -- -- Serum Urea Nitrogen 12 4 0 -- -- -- -- 2 0 2 SGOT 63 24 4 12 11 41 -- -- -- -- SGPT 2 -- 13 -- 10 15 -- -- -- -- Granulocyte Count - Total
92 36 NA -- 31 39 45Þ 75Þ 61Þ 70Þ - 1000-<1500/mm3
66 -- -- -- -- -- 32 30 32 43 - 750-<1000/mm3
-- 21 -- -- -- -- 10 24 18 18 - 500-<750/mm3
25 -- -- -- -- -- 1 17 9 7 - <500/mm3
1 13 -- -- -- -- 2 4 2 2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of INTRON A alone or in combination with REBETOL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders
pancytopenia (concurrent anemia, leukopenia, thrombocytopenia), aplastic anemia, pure red cell aplasia, thrombotic thrombocytopenic purpura, idiopathic thrombocytopenic purpura
Cardiac Disorders
pericarditis
Ear and Labyrinth Disorders
hearing loss
Endocrine Disorders
hypopituitarism
Eye Disorders
Vogt-Koyanagi-Harada syndrome, serous retinal detachment
Gastrointestinal Disorders
pancreatitis, tongue pigmentation
General Disorders and Administration Site Conditions
asthenic conditions (including asthenia, malaise, fatigue)
Immune System Disorders
cases of acute hypersensitivity reactions, including anaphylaxis and angioedema, systemic lupus erythematosus, sarcoidosis or exacerbation of sarcoidosis
Infections and Infestations
hepatitis B virus reactivation in HCV/HBV co-infected patients
Musculoskeletal and Connective Tissue Disorders
myositis
Nervous System Disorders
peripheral neuropathy
Psychiatric Disorders
homicidal ideation, psychosis including hallucinations
Renal and Urinary Disorders
renal failure, renal insufficiency, nephrotic syndrome
Respiratory, Thoracic, and Mediastinal Disorders
pulmonary hypertension, pulmonary fibrosis
Skin and Subcutaneous Tissue Disorders
injection site necrosis, Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme, urticaria
-
OVERDOSAGE
There is limited experience with overdosage. Postmarketing surveillance includes reports of patients receiving a single dose as great as 10 times the recommended dose. In general, the primary effects of an overdose are consistent with the effects seen with therapeutic doses of interferon alfa-2b. Hepatic enzyme abnormalities, renal failure, hemorrhage, and myocardial infarction have been reported with single administration overdoses and/or with longer durations of treatment than prescribed (see ADVERSE REACTIONS). Toxic effects after ingestion of interferon alfa-2b are not expected because interferons are poorly absorbed orally. Consultation with a poison center is recommended.
-
DOSAGE AND ADMINISTRATION
General
IMPORTANT: INTRON® A is supplied as 1) Powder for Injection/Reconstitution; 2) Solution for Injection in Vials. Not all dosage forms and strengths are appropriate for some indications. It is important that you carefully read the instructions below for the indication you are treating to ensure you are using an appropriate dosage form and strength.
To enhance the tolerability of INTRON A, injections should be administered in the evening when possible.
To reduce the incidence of certain adverse reactions, acetaminophen may be administered at the time of injection.
The solution should be allowed to come to room temperature before using.
Hairy Cell Leukemia
(see DOSAGE AND ADMINISTRATION, General)
Dose
The recommended dose for the treatment of hairy cell leukemia is 2 million IU/m2 administered intramuscularly or subcutaneously 3 times a week for up to 6 months. Patients with platelet counts of less than 50,000/mm3 should not be administered INTRON A intramuscularly, but instead by subcutaneous administration. Patients who are responding to therapy may benefit from continued treatment.
Dosage Forms for This Indication Dosage Form Concentration Route Fixed Doses Powder 10 MIU (single dose) 10 MIU/mL IM, SC N/A Solution 18 MIU multidose 6 MIU/mL IM, SC N/A Solution 25 MIU multidose 10 MIU/mL IM, SC N/A NOTE: INTRON A Powder for Injection does not contain a preservative. The vial must be discarded after reconstitution and withdrawal of a single dose.
Dose Adjustment
- If severe adverse reactions develop, the dosage should be modified (50% reduction) or therapy should be temporarily withheld until the adverse reactions abate and then resume at 50% (1 MIU/m2 TIW).
- If severe adverse reactions persist or recur following dosage adjustment, INTRON A should be permanently discontinued.
- INTRON A should be discontinued for progressive disease or failure to respond after six months of treatment.
Malignant Melanoma
(see DOSAGE AND ADMINISTRATION, General)
INTRON A adjuvant treatment of malignant melanoma is given in two phases, induction and maintenance.
Induction Recommended Dose
The recommended daily dose of INTRON A in induction is 20 million IU/m2 as an intravenous infusion, over 20 minutes, 5 consecutive days per week, for 4 weeks (see Dose Adjustment below).
Dosage Forms for This Indication Dosage Form Concentration Route Powder 10 MIU 10 MIU/mL IV Powder 18 MIU 18 MIU/mL IV Powder 50 MIU 50 MIU/mL IV NOTE: INTRON A Solution for Injection in vials is NOT recommended for intravenous administration and should not be used for the induction phase of malignant melanoma.
NOTE: INTRON A Powder for Injection does not contain a preservative. The vial must be discarded after reconstitution and withdrawal of a single dose.
Dose Adjustment
NOTE: Regular laboratory testing should be performed to monitor laboratory abnormalities for the purpose of dose modifications (see PRECAUTIONS, Laboratory Tests).
- INTRON A should be withheld for severe adverse reactions, including granulocyte counts greater than 250/mm3 but less than 500/mm3 or SGPT/SGOT greater than 5-10× upper limit of normal, until adverse reactions abate. INTRON A treatment should be restarted at 50% of the previous dose.
- INTRON A should be permanently discontinued for:
- Toxicity that does not abate after withholding INTRON A
- Severe adverse reactions which recur in patients receiving reduced doses of INTRON A
- Granulocyte count less than 250/mm3 or SGPT/SGOT of greater than 10× upper limit of normal
Maintenance Recommended Dose
The recommended dose of INTRON A for maintenance is 10 million IU/m2 as a subcutaneous injection three times per week for 48 weeks (see Dose Adjustment below).
Dosage Forms for This Indication Dosage Form Concentration Route Fixed Doses - * Patients receiving 50% dose reduction only
- † Patients receiving full dose only
Powder 10 MIU (single dose)* 10 MIU/mL SC N/A Powder 18 MIU (single dose)† 18 MIU/mL SC N/A Solution 18 MIU multidose 6 MIU/mL SC N/A Solution 25 MIU multidose 10 MIU/mL SC N/A NOTE: INTRON A Powder for Injection does not contain a preservative. The vial must be discarded after reconstitution and withdrawal of a single dose.
Dose Adjustment
NOTE: Regular laboratory testing should be performed to monitor laboratory abnormalities for the purpose of dose modifications (see PRECAUTIONS, Laboratory Tests).
- INTRON A should be withheld for severe adverse reactions, including granulocyte counts greater than 250/mm3 but less than 500/mm3 or SGPT/SGOT greater than 5-10× upper limit of normal, until adverse reactions abate. INTRON A treatment should be restarted at 50% of the previous dose.
- INTRON A should be permanently discontinued for:
- Toxicity that does not abate after withholding INTRON A
- Severe adverse reactions which recur in patients receiving reduced doses of INTRON A
- Granulocyte count less than 250/mm3 or SGPT/SGOT of greater than 10× upper limit of normal
Follicular Lymphoma
(see DOSAGE AND ADMINISTRATION, General)
Dose
The recommended dose of INTRON A for the treatment of follicular lymphoma is 5 million IU subcutaneously three times per week for up to 18 months in conjunction with anthracycline-containing chemotherapy regimen and following completion of the chemotherapy regimen.
Dosage Forms for This Indication Dosage Form Concentration Route Fixed Doses Powder 10 MIU (single dose) 10 MIU/mL SC N/A Solution 18 MIU multidose 6 MIU/mL SC N/A Solution 25 MIU multidose 10 MIU/mL SC N/A NOTE: INTRON A Powder for Injection does not contain a preservative. The vial must be discarded after reconstitution and withdrawal of a single dose.
Dose Adjustment
- Doses of myelosuppressive drugs were reduced by 25% from a full-dose CHOP regimen, and cycle length increased by 33% (e.g., from 21 to 28 days) when alpha interferon was added to the regimen.
- Delay chemotherapy cycle if neutrophil count was less than 1500/mm3 or platelet count was less than 75,000/mm3.
- INTRON A should be permanently discontinued if SGOT exceeds greater than 5× the upper limit of normal or serum creatinine greater than 2.0 mg/dL (see WARNINGS).
- Administration of INTRON A therapy should be withheld for a neutrophil count less than 1000/mm3, or a platelet count less than 50,000/mm3.
- INTRON A dose should be reduced by 50% (2.5 MIU TIW) for a neutrophil count greater than 1000/mm3, but less than 1500/mm3. The INTRON A dose may be re-escalated to the starting dose (5 million IU TIW) after resolution of hematologic toxicity (ANC greater than 1500/mm3).
Condylomata Acuminata
(see DOSAGE AND ADMINISTRATION, General)
Dose
The recommended dose is 1.0 million IU per lesion in a maximum of 5 lesions in a single course. The lesions should be injected three times weekly on alternate days for 3 weeks. An additional course may be administered at 12 to 16 weeks.
Dosage Forms for This Indication Dosage Form Concentration Route Powder 10 MIU (single dose) 10 MIU/mL IL Solution 25 MIU multidose 10 MIU/mL IL NOTE: INTRON A Powder for Injection does not contain a preservative. The vial must be discarded after reconstitution and withdrawal of a single dose.
NOTE: Do not use the following formulations for this indication:
- the 18 million or 50 million IU Powder for Injection
- the 18 million IU multidose INTRON A Solution for Injection
Technique for Injection
The injection should be administered intralesionally using a Tuberculin or similar syringe and a 25- to 30-gauge needle. The needle should be directed at the center of the base of the wart and at an angle almost parallel to the plane of the skin (approximately that in the commonly used PPD test). This will deliver the interferon to the dermal core of the lesion, infiltrating the lesion and causing a small wheal. Care should be taken not to go beneath the lesion too deeply; subcutaneous injection should be avoided, since this area is below the base of the lesion. Do not inject too superficially since this will result in possible leakage, infiltrating only the keratinized layer and not the dermal core.
AIDS-Related Kaposi's Sarcoma
(see DOSAGE AND ADMINISTRATION, General)
Dose
The recommended dose of INTRON A for Kaposi's Sarcoma is 30 million IU/m2/dose administered subcutaneously or intramuscularly three times a week until disease progression or maximal response has been achieved after 16 weeks of treatment. Dose reduction is frequently required (see Dose Adjustment below).
Dosage Forms for This Indication Dosage Form Concentration Route Powder 50 MIU 50 MIU/mL IM, SC NOTE: INTRON A Solution for Injection in vials should NOT be used for AIDS-Related Kaposi's Sarcoma.
NOTE: INTRON A Powder for Injection does not contain a preservative. The vial must be discarded after reconstitution and withdrawal of a single dose.
Dose Adjustment
- INTRON A dose should be reduced by 50% or withheld for severe adverse reactions.
- INTRON A may be resumed at a reduced dose if severe adverse reactions abate with interruption of dosing.
- INTRON A should be permanently discontinued if severe adverse reactions persist or if they recur in patients receiving a reduced dose.
Chronic Hepatitis C
(see DOSAGE AND ADMINISTRATION, General)
Dose
The recommended dose of INTRON A for the treatment of chronic hepatitis C is 3 million IU three times a week (TIW) administered subcutaneously or intramuscularly. In patients tolerating therapy with normalization of ALT at 16 weeks of treatment, INTRON A therapy should be extended to 18 to 24 months (72 to 96 weeks) at 3 million IU TIW to improve the sustained response rate (see CLINICAL PHARMACOLOGY, Chronic Hepatitis C). Patients who do not normalize their ALTs or have persistently high levels of HCV RNA after 16 weeks of therapy rarely achieve a sustained response with extension of treatment. Consideration should be given to discontinuing these patients from therapy.
When INTRON A is administered in combination with REBETOL®, patients with impaired renal function and/or those over the age of 50 should be carefully monitored with respect to the development of anemia. See REBETOL prescribing information for dosing when used in combination with REBETOL for adults and pediatric patients.
Dosage Forms for This Indication Dosage Form Concentration Route Fixed Doses Solution 18 MIU multidose 6 MIU/mL IM, SC N/A Chronic Hepatitis B Adults
(see DOSAGE AND ADMINISTRATION, General)
Dose
The recommended dose of INTRON A for the treatment of chronic hepatitis B is 30 to 35 million IU per week, administered subcutaneously or intramuscularly, either as 5 million IU daily (QD) or as 10 million IU three times a week (TIW) for 16 weeks.
Dosage Forms for This Indication Dosage Form Concentration Route Fixed Doses Powder 10 MIU (single dose) 10 MIU/mL IM, SC N/A Solution 25 MIU multidose 10 MIU/mL IM, SC N/A NOTE: INTRON A Powder for Injection does not contain a preservative. The vial must be discarded after reconstitution and withdrawal of a single dose.
Chronic Hepatitis B Pediatrics
(see DOSAGE AND ADMINISTRATION, General)
Dose
The recommended dose of INTRON A for the treatment of chronic hepatitis B is 3 million IU/m2 three times a week (TIW) for the first week of therapy followed by dose escalation to 6 million IU/m2 TIW (maximum of 10 million IU TIW) administered subcutaneously for a total duration of 16 to 24 weeks.
Dosage Forms for This Indication Dosage Form Concentration Route Fixed Doses Powder 10 MIU (single dose) 10 MIU/mL SC N/A Solution 25 MIU multidose 10 MIU/mL SC N/A NOTE: INTRON A Powder for Injection does not contain a preservative. The vial must be discarded after reconstitution and withdrawal of a single dose.
Dose Adjustment
If severe adverse reactions or laboratory abnormalities develop during INTRON A therapy, the dose should be modified (50% reduction) or discontinued if appropriate, until the adverse reactions abate. If intolerance persists after dose adjustment, INTRON A therapy should be discontinued.
For patients with decreases in white blood cell, granulocyte or platelet counts, the following guidelines for dose modification should be followed:
INTRON A Dose White Blood Cell Count Granulocyte Count Platelet Count Reduce 50% <1.5 × 109/L <0.75 × 109/L <50 × 109/L Permanently Discontinue <1.0 × 109/L <0.5 × 109/L <25 × 109/L INTRON A therapy was resumed at up to 100% of the initial dose when white blood cell, granulocyte, and/or platelet counts returned to normal or baseline values.
-
PREPARATION AND ADMINISTRATION
Reconstitution of INTRON® A Powder for Injection
Reconstitute INTRON A Powder for Injection with 1 mL of Sterile Water for Injection, USP. The Sterile Water for Injection supplied contains 5 mL and is intended for single-dose. Discard the unused portion. The reconstituted solution is clear and colorless to light yellow. The INTRON A powder reconstituted with Sterile Water for Injection USP is a single-dose vial and does not contain a preservative. DO NOT RE-ENTER VIAL AFTER WITHDRAWING THE DOSE. DISCARD UNUSED PORTION (see DOSAGE AND ADMINISTRATION). Once the dose from the single-dose vial has been withdrawn, the sterility of any remaining product can no longer be guaranteed. Pooling of unused portions of some medications has been linked to bacterial contamination and morbidity.
Intramuscular, Subcutaneous, or Intralesional Administration
Inject 1 mL Diluent (Sterile Water for Injection USP) for INTRON A into the INTRON A vial. Swirl gently to hasten complete dissolution of the powder. The appropriate INTRON A dose should then be withdrawn and injected intramuscularly, subcutaneously, or intralesionally (see MEDICATION GUIDE and Instructions for Use for detailed instructions).
Please refer to the MEDICATION GUIDE and Instructions for Use for detailed, step-by-step instructions on how to inject the INTRON A dose. After preparation and administration of the INTRON A injection, it is essential to follow the procedure for proper disposal of syringes and needles (see MEDICATION GUIDE and Instructions for Use for detailed instructions).
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Intravenous Infusion
The infusion solution should be prepared immediately prior to use. Based on the desired dose, the appropriate vial strength(s) of INTRON A should be reconstituted with the diluent provided. Inject 1 mL Diluent (Sterile Water for Injection USP) for INTRON A into the INTRON A vial. Swirl gently to hasten complete dissolution of the powder. The appropriate INTRON A dose should then be withdrawn and injected into a 100-mL bag of 0.9% Sodium Chloride Injection USP. The final concentration of INTRON A should not be less than 10 million IU/100 mL.
INTRON A Solution for Injection in Vials
INTRON A Solution for Injection is supplied in two multidose vials. The solutions for injection do not require reconstitution prior to administration; the solution is clear and colorless.
The appropriate dose should be withdrawn from the vial and injected intramuscularly, subcutaneously, or intralesionally.
INTRON A Solution for Injection is not recommended for intravenous administration.
Please refer to the MEDICATION GUIDE and Instructions for Use for detailed, step-by-step instructions on how to inject the INTRON A dose. After preparation and administration of INTRON A, it is essential to follow the procedure for proper disposal of syringes and needles.
-
HOW SUPPLIED
INTRON® A Powder for Injection
INTRON A Powder for Injection, 10 million IU per vial and Diluent for INTRON A (Sterile Water for Injection USP) 5 mL per vial; boxes containing 1 INTRON A vial and 1 vial of INTRON A Diluent (NDC: 0085-4350-01).
INTRON A Powder for Injection, 18 million IU per vial and Diluent for INTRON A (Sterile Water for Injection USP) 5 mL per vial; boxes containing 1 vial of INTRON A and 1 vial of INTRON A Diluent (NDC: 0085-4351-01).
INTRON A Powder for Injection, 50 million IU per vial and Diluent for INTRON A (Sterile Water for Injection USP) 5 mL per vial; boxes containing 1 INTRON A vial and 1 vial of INTRON A Diluent (NDC: 0085-4352-01).
INTRON A Solution for Injection in Vials
INTRON A Solution for Injection, 18 million IU multidose vial (22.8 million IU per 3.8 mL per vial); boxes containing 1 vial of INTRON A Solution for Injection (NDC: 0085-1168-01).
INTRON A Solution for Injection, 25 million IU multidose vial (32 million IU per 3.2 mL per vial); boxes containing 1 vial of INTRON A Solution for Injection (NDC: 0085-1133-01).
Storage
-
INTRON A Powder for Injection/Reconstitution
INTRON A Powder for Injection should be stored in the refrigerator at 2° to 8°C (36°-46°F). After reconstitution, the solution should be used immediately, but may be stored up to 24 hours at 2° to 8°C (36°-46°F). Throw away any medicine left in the vial after you withdraw 1 dose. -
INTRON A Solution for Injection in Vials
INTRON A Solution for Injection in vials should be stored in the refrigerator at 2° to 8°C (36°-46°F).
INTRON A Solution for Injection should not be frozen and should be kept away from heat. Throw away any unused INTRON A Solution for Injection remaining in the vial after one month.
-
INTRON A Powder for Injection/Reconstitution
-
References
- Smalley R, et al. N Engl J Med. 1992;327:1336-1341.
- Aviles A, et al. Leukemia and Lymphoma. 1996;20:495-499.
- Unterhalt M, et al. Blood. 1996;88(10 Suppl 1):1744A.
- Schiller J, et al. J Biol Response Mod. 1989;8:252-261.
- Poynard T, et al. N Engl J Med. 1995;332(22)1457-1462.
- Lin R, et al. J Hepatol. 1995;23:487-496.
- Perrillo R, et al. N Engl J Med. 1990;323:295-301.
- Perez V, et al. J Hepatol. 1990;11:S113-S117.
- Knodell R, et al. Hepatology. 1981;1:431-435.
- Perrillo R, et al. Ann Intern Med. 1991;115:113-115.
- Kauppila A, et al. Int J Cancer. 1982;29:291-294.
-
SPL UNCLASSIFIED SECTION
Manufactured by:
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
U.S. License Number 0002For patent information: www.merck.com/product/patent/home.html
Copyright © 1986-2019 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.Rev.08/2019
uspi-mk2958-mf-5ml-1908r006
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 08/2019 MEDICATION GUIDE
INTRON® A (In-tron-aye)
(Interferon alfa-2b, recombinant)If you are taking INTRON A with REBETOL, also read the Medication Guide for REBETOL® (ribavirin) Capsules and Oral Solution.
INTRON A alone is a treatment for certain types of cancers and hepatitis B virus. INTRON A by itself or with REBETOL is a treatment for some people infected with hepatitis C virus.What is the most important information I should know about INTRON A?
INTRON A can cause serious side effects that may cause death or worsen certain serious conditions that you may already have.
Tell your healthcare provider right away if you have any of the symptoms listed below while taking INTRON A. If symptoms get worse, or become severe and continue, your healthcare provider may tell you to stop taking INTRON A permanently. In many, but not all people, these symptoms go away after they stop taking INTRON A.
Heart problems. Some people who take INTRON A may develop heart problems, including:- low blood pressure
- fast heart rate or abnormal heart beats
- trouble breathing or chest pain
- heart attacks or heart muscle problems (cardiomyopathy)
Stroke or symptoms of a stroke. Symptoms may include weakness, loss of coordination, and numbness. Stroke or symptoms of a stroke may happen in people who have some risk factors or no known risk factors for a stroke.
Mental health problems, including suicide. INTRON A may cause you to develop mood or behavior problems that may get worse during treatment with INTRON A or after your last dose, including:- irritability (getting upset easily)
- depression (feeling low, feeling bad about yourself, or feeling hopeless)
- acting aggressive, being angry or violent
- thoughts of hurting yourself or others, or suicide
- former drug addicts may fall back into drug addiction or overdose
If you have these symptoms, your healthcare provider should carefully monitor you during treatment with INTRON A and for 6 months after your last dose.
New or worsening autoimmune disease. Some people taking INTRON A develop autoimmune diseases (a condition where the body's immune cells attack other cells or organs in the body), including rheumatoid arthritis, systemic lupus erythematosus, sarcoidosis, and psoriasis. In some people who already have an autoimmune disease, the disease may get worse while on INTRON A.
Infections. Some people who take INTRON A may get an infection. Symptoms may include:- fever
- chills
- bloody diarrhea
- burning or pain with urination
- urinating often
- coughing up mucus (phlegm) that is colored (for example yellow or pink)
During treatment with INTRON A, you should see a healthcare provider regularly for check-ups and blood tests to make sure that your treatment is working, and to check for side effects. What is INTRON A?
INTRON A is a prescription medicine that is used:- to treat adults with a blood cancer called hairy cell leukemia
- to treat certain adults with a type of skin cancer called malignant melanoma
- to treat adults with some types of Follicular Non-Hodgkin's Lymphoma along with certain chemotherapy medicines
- to treat certain adults with genital warts (condylomata acuminata), by injecting the medicine directly into the warts
- to treat certain adults with a type of cancer caused by AIDS, called AIDS-related Kaposi's Sarcoma
- alone to treat adults with chronic (lasting a long time) hepatitis C infection with stable liver problems
- with REBETOL to treat chronic (lasting a long time) hepatitis C infection in people 3 years and older with stable liver problems
- to treat chronic (lasting a long time) hepatitis B infection in people 1 year and older with stable liver problems
Who should not take INTRON A?
Do not take INTRON A if you:- had a serious allergic reaction to another alpha interferon product or are allergic to any of the ingredients in INTRON A. See the end of this Medication Guide for a complete list of ingredients. Ask your healthcare provider if you are not sure.
- have certain types of hepatitis (autoimmune hepatitis)
- have certain other liver problems
What should I tell my healthcare provider before taking INTRON A?
Before you take INTRON A, tell your healthcare provider about all of your health problems, including if you:- See "What is the most important information I should know about INTRON A?"
- have or ever had any problems with your heart, including heart attack or have high blood pressure
- have or ever had bleeding problems or blood clots
- are being treated for a mental illness or had treatment in the past for any mental illness, including depression and thoughts of hurting yourself or others
- have any kind of autoimmune disease (where the body's immune system attacks the body's own cells), such as psoriasis, systemic lupus erythematosus, rheumatoid arthritis
- have or ever had low blood cell counts
- have ever been addicted to drugs or alcohol
- have cirrhosis or other liver problems (other than hepatitis B or C)
- have or had lung problems, such as chronic obstructive pulmonary disease (COPD)
- have diabetes
- have colitis (inflammation of your intestine)
- have a condition that suppresses your immune system, such as cancer
- have hepatitis B or C infection
- have HIV infection (the virus that causes AIDS)
- have kidney problems
- have high blood triglyceride levels (fat in your blood)
- have an organ transplant and are taking medicine that keeps your body from rejecting your transplant (suppresses your immune system)
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if INTRON A will harm your unborn baby. You should use effective birth control during treatment with INTRON A. Talk to your healthcare provider about birth control choices for you during treatment with INTRON A. Tell your healthcare provider if you become pregnant during treatment with INTRON A.
- are breast-feeding or plan to breast-feed. It is not known if INTRON A passes into your breast milk. You and your healthcare provider should decide if you will use INTRON A or breast-feed. You should not do both.
Especially tell your healthcare provider if you take:- the anti-hepatitis B medicine telbivudine (Tyzeka)
- the anti-HIV medicine zidovudine (Retrovir)
- theophylline (Theo-24, Elixophyllin, Uniphyl, Theolair). Your healthcare provider may need to monitor the amount of theophylline in your body and make changes to your theophylline dose.
How should I take INTRON A? - See the attached Instructions for Use for detailed instructions for preparing and injecting a dose of INTRON A.
- INTRON A comes as:
- a powder for injection in a vial that is used only 1 time (single-dose vial). The powder must be mixed with water for injection (a diluent) before you inject it.
- a solution for injection in a multi-dose vial.
- INTRON A is given as an injection under the skin (subcutaneous) or into a muscle (intramuscular), into genital lesions, or as an injection into a vein (intravenous), depending on the condition that is being treated.
- Your healthcare provider will decide your dose of INTRON A and how often you will take it.
- If your healthcare provider decides that you can inject INTRON A for your condition, inject it exactly as prescribed, under your skin (subcutaneous injection) or into your muscle (intramuscular injection). Do not change your dose or how you inject INTRON A unless your healthcare provider tells you to.
- Do not take more than your prescribed dose.
- Your healthcare provider should show you how to prepare and measure your dose of INTRON A and how to inject yourself before you use INTRON A for the first time.
- You should not inject INTRON A until your healthcare provider has shown you how to use INTRON A the right way.
- If you miss a dose of INTRON A, take the missed dose as soon as possible during the same day or the next day, then continue on your regular dosing schedule. If several days go by after you miss a dose, check with your healthcare provider to see what to do.
- Do not inject more than 1 dose or take more than your prescribed dose without talking to your healthcare provider.
- If you take too much INTRON A, call your healthcare provider right away. Your healthcare provider may examine you more closely, and do blood tests.
- Your healthcare provider should do blood tests before you start INTRON A, and regularly during your treatment to see how well the treatment is working, and to check for side effects.
What are the possible side effects of INTRON A?
INTRON A may cause serious side effects including:- See "What is the most important information I should know about INTRON A?"
- Blood problems. INTRON A can affect your bone marrow and cause low white blood cell and platelet counts. In some people, these blood counts may fall to dangerously low levels. If your blood cell counts become very low, you can get infections or have bleeding problems.
- Serious eye problems. INTRON A may cause eye problems that may lead to vision loss or blindness. You should have an eye exam before you start taking INTRON A. If you have eye problems or have had them in the past, you may need eye exams while taking INTRON A. Tell your healthcare provider or eye doctor right away if you have any vision changes while taking INTRON A.
- Thyroid problems. Some people develop changes in the function of their thyroid. Symptoms of thyroid problems include:
- problems concentrating
- feeling cold or hot all the time
- changes in your weight
- skin changes
- Blood sugar problems. Some people may develop high blood sugar or diabetes. If you have high blood sugar or diabetes before starting INTRON A, talk to your healthcare provider before you take INTRON A. If you develop high blood sugar or diabetes while taking INTRON A, your healthcare provider may tell you to stop INTRON A and prescribe a different medicine for you. Symptoms of high blood sugar or diabetes may include:
- increased thirst
- tiredness
- urinating more often than normal
- increased appetite
- weight loss
- your breath smells like fruit
- Lung problems including:
- trouble breathing
- pneumonia
- inflammation of lung tissue
- new or worse high blood pressure of the lungs (pulmonary hypertension). This can be severe and may lead to death.
You may need to have a chest X-ray or other tests if you develop fever, cough, shortness of breath, or other symptoms of a lung problem during treatment with INTRON A. - Severe liver problems, or worsening of liver problems including liver failure and death. Symptoms may include:
- nausea
- loss of appetite
- tiredness
- diarrhea
- yellowing of your skin or the white part of your eyes
- bleeding more easily than normal
- swelling of your stomach area (abdomen)
- confusion
- sleepiness
- you cannot be awakened (coma)
- Serious allergic reactions and skin reactions. Symptoms may include:
- itching
- swelling of your face, eyes, lips, tongue, or throat
- trouble breathing
- anxiousness
- chest pain
- feeling faint
- skin rash, hives, sores in your mouth, or your skin blisters and peels
- Swelling of your pancreas (pancreatitis) and intestines (colitis). Symptoms may include:
- severe stomach area (abdomen) pain
- severe back pain
- nausea
- vomiting
- fever
- New or worsening autoimmune disease. Some people taking INTRON A develop autoimmune diseases (a condition where the body's immune cells attack other cells or organs in the body), including rheumatoid arthritis, systemic lupus erythematosus, sarcoidosis, and psoriasis. In some people who already have an autoimmune disease, the disease may worsen while on INTRON A.
- Nerve problems. People who take INTRON A or other alpha interferon products with telbivudine (Tyzeka) can develop nerve problems such as continuing numbness, tingling, or burning sensation in the arms or legs (peripheral neuropathy). Call your healthcare provider if you have any of these symptoms.
- Growth problems in children. Weight loss and slowed growth are common in children during combination treatment with INTRON A and REBETOL. Most children will go through a growth spurt and gain weight after treatment stops. Some children may not reach the height that they were expected to have before treatment. Talk to your healthcare provider if you are concerned about your child's growth during treatment with INTRON A and REBETOL.
- Dental and gum problems.
The most common side effects of INTRON A include:- Flu-like symptoms. Symptoms may include: headache, muscle aches, tiredness, and fever. Some of these symptoms may be decreased by injecting your INTRON A dose in the evening. Talk to your healthcare provider about which over-the-counter medicines you can take to help prevent or decrease some of the symptoms.
- Tiredness. Many people become very tired during treatment with INTRON A.
- Appetite problems. Nausea, loss of appetite, and weight loss can happen with INTRON A.
- Skin reactions. Redness, swelling, and itching are common at the injection site.
- Hair thinning.
These are not all the side effects of INTRON A. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1–800–FDA–1088.How should I store INTRON A?
INTRON A Solution for Injection:- Store in the refrigerator between 36°F to 46°F (2°C to 8°C).
- INTRON A Solution for Injection in Multidose vials for injection may be used to give more than 1 injection of medicine.
- Do not freeze.
- Throw away any unused INTRON A Solution for Injection remaining in the vial after one month.
Before mixing, store in the refrigerator between 36°F to 46°F (2°C to 8°C).- After mixing the INTRON A Powder for Injection, use the solution right away or store the solution in the refrigerator for up to 24 hours between 36°F to 46°F (2°C to 8°C).
- Throw away any medicine left in the vial after you withdraw 1 dose.
- Do not freeze.
General Information about the safe and effective use of INTRON A
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use INTRON A for a condition for which it was not prescribed. Do not give INTRON A to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about INTRON A. If you would like more information, ask your healthcare provider. You can ask your healthcare provider or pharmacist for information about INTRON A that was written for health care professionals.What are the ingredients in INTRON A?
Active ingredient: interferon alfa-2b
Inactive ingredients:- Powder for injection contains: glycine, sodium phosphate dibasic, sodium phosphate monobasic, human albumin. Sterile water for injection is provided as a diluent.
- Solution Multidose vials for injection contain: sodium chloride, sodium phosphate dibasic, sodium phosphate monobasic, edetate disodium, polysorbate 80, and m-cresol as a preservative.
Manufactured by:
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
U.S. License Number 0002
Copyright © 1996-2019 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.
usmg-mk2958-mf-1908r014
For more information, go to www.IntronA.com or call 1-800-622-4477. -
Instructions for Use
INTRON® A (In-tron-aye)
(Interferon alfa-2b, recombinant)
Solution for InjectionBe sure that you read, understand and follow these instructions before injecting INTRON A. Your healthcare provider should show you how to prepare, measure and inject INTRON A properly before you use it for the first time. Ask your healthcare provider if you have any questions.
Before starting, collect all of the supplies that you will need to use for preparing and injecting INTRON A. For each injection, you will need the following supplies:
- 1 vial of INTRON A solution
- 1 single-dose disposable syringe and needle
- 1 cotton ball or gauze
- 2 alcohol swabs
- 1 sharps disposal container for throwing away (dispose of) your used syringes, needles, and vials. See "How should I dispose of used syringes, needles, and vials?" at the end of this Instructions for Use.
Important:
- Never re-use disposable syringes and needles.
- Make sure you have the right syringe and needle to use with INTRON A. Your healthcare provider should tell you what syringes and needles to use to inject INTRON A.
How should I prepare a dose of INTRON® A?
- 1 Find a well lit, clean, flat working surface.
- 2. Before removing INTRON A from the carton, look at the expiration date printed on the carton. Make sure that the expiration date has not passed. Do not use if the expiration date has passed.
- 3. Wash your hands well with soap and warm water (See Figure A). Keep your work area, your hands and injection site clean to decrease the risk of infection.
- 4. Remove 1 vial of INTRON A solution from the carton (See Figure B).
- 5. Look at the vial of INTRON A. The solution should be clear and colorless, without particles. Do not use the vial of INTRON A if the medicine is cloudy, has particles or is not clear and colorless.
- 6. Remove the protective plastic cap from the top of the INTRON A vial. Clean the rubber stopper on the top of the INTRON A vial with an alcohol swab (See Figure C).
- 7. Gently warm the INTRON A solution by slowly rolling the vial in the palms of your hands for about one minute (See Figure D). Do not shake the vial.
- 8. Open the package of the syringe you are using (See Figure E) and if it does not have a needle attached, then attach a new needle to the syringe.
- 9. Remove the protective cap from the needle of the syringe. Fill the syringe with air by pulling back on the plunger to the mark on the syringe that matches the dose prescribed by your healthcare provider (See Figure F).
- 10. Hold the vial of INTRON A Solution for Injection on your flat working surface (See Figure G). Do not touch the cleaned rubber stopper.
- 11. Push the needle straight down through the middle of the rubber stopper of the vial containing the INTRON A solution (See Figure H). Slowly inject all the air from the syringe into the air space above the solution.
- 12.
Keep the needle in the vial. Turn the vial upside down (See Figure I).
- Make sure the tip of the needle is in the INTRON A solution.
- Slowly pull the plunger back to fill the syringe with INTRON A solution to the dose (mL or cc) prescribed by your healthcare provider.
- 13.
With the needle still in the vial, check the syringe for air bubbles (See Figure J).
- If there are any air bubbles, gently tap the syringe with your finger until the air bubbles rise to the top of the syringe.
- Slowly push the plunger up to remove the air bubbles.
- If you push solution back into the vial, slowly pull back on the plunger to draw the dose (mL or cc) prescribed by your healthcare provider.
- 14. Do not remove the needle from the vial. Lay the vial and syringe on its side on your flat work surface until you are ready to inject the INTRON A solution.
How should I choose a site for injection?
Based on your treatment, your healthcare provider will tell you if you should inject a dose of INTRON® A under the skin (subcutaneous injection) or into the muscle (intramuscular injection). If it is too difficult for you to inject, ask someone who has been trained to give injections to help you.
For Subcutaneous Injection
The best sites for injection are areas on your body with a layer of fat between skin and muscle such as (See Figure K):
- the front of your middle thighs
- the outer area of your upper arms
- the abdomen, except around your belly button (navel)
For Intramuscular Injection
The best sites for injection into your muscle are (See Figure L):
- the front of your middle thighs
- your upper arms
- the upper outer areas of your buttocks
You should use a different site each time you inject INTRON® A to avoid soreness at any one site. Do not inject INTRON A into an area where the skin is irritated, red, bruised, infected or has scars, stretch marks or lumps.
How should I inject a dose of INTRON® A?
- 15. Clean the injection site with a new alcohol swab. Wait for the area to dry.
- 16.
Pick up the vial and syringe from your flat work surface. Remove the syringe and needle from the vial.
- Hold the syringe in the hand that you will use to inject INTRON A.
- Do not touch the needle or allow it to touch the work surface.
- 17. With your other hand, pinch a fold of the skin at the cleaned injection site.
For subcutaneous injection (under the skin):
- Hold the syringe (like a pencil) at a 45-degree angle to the skin. With a quick "dart-like" motion, push the needle into the skin (See Figure M).
For intramuscular injection (into the muscle):
- Hold the syringe (like a pencil) at a 90-degree angle to the skin. With a quick "dart-like" motion, push the needle into the muscle (See Figure N).
- 18.
After the needle is inserted, remove the hand used to pinch the skin. Use it to hold the syringe barrel.
- Pull the plunger back slightly.
- If no blood is present in the syringe, inject the medicine by gently pressing the plunger all the way down the syringe barrel, until the syringe is empty.
-
If blood comes into the syringe, the needle has entered a blood vessel. Do not inject INTRON® A.
- Withdraw the needle and dispose of the syringe and needle in the sharps disposal container. See "How should I dispose of used syringes, needles and vials?" at the end of this Instructions for Use
- If there is bleeding, cover the injection site with a bandage.
- Then, repeat steps 1 through 18 with a new dose of INTRON A and inject the medicine at a new injection site.
- 19.
When the syringe is empty, pull the needle out of the skin.
- Place a cotton ball or gauze over the injection site and press for several seconds. Do not massage the injection site.
- If there is bleeding, cover the injection site with a bandage.
- 20. Dispose of used syringes, needles and vials in the sharps disposal container. See "How should I dispose of used syringes, needles, and vials?" below.
How should I dispose of used syringes, needles, and vials?
- Put your used needles, syringes and vials in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles, syringes and vials in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used syringes and needles. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Always keep the sharps disposal container out of the reach of children.
How should I store INTRON® A?
- Store in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze Intron A.
- Allow the INTRON A Solution for Injection to come to room temperature before using. INTRON A Solution in Multidose vials for Injection may be used to give more than 1 injection of medicine.
- Throw away any unused INTRON A Solution for Injection remaining in the vial after 1 month
- Keep away from heat.
- Keep INTRON A and all medicines out of the reach of children.
-
SPL UNCLASSIFIED SECTION
Manufactured by:
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
U.S. License Number 0002For patent information: www.merck.com/product/patent/home.html
Copyright © 1996- 2019, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.Rev.08/2019
usifu-mk2958-soi-1908r008
-
Instructions for Use
INTRON® A (In-tron-aye)
(Interferon alfa-2b, recombinant)
Powder for SolutionBe sure that you read, understand, and follow these instructions before injecting INTRON A. Your healthcare provider should show you how to prepare, measure, and inject INTRON A properly before you use it for the first time. Ask your healthcare provider if you have any questions.
Before starting, collect all of the supplies that you will need to use for preparing and injecting INTRON A. For each injection you will need the following supplies:
- 1 vial of INTRON A powder for solution
- 1 vial of sterile water for injection (diluent). The vial contains an excess amount of sterile water (5 mL). You will only need to withdraw 1 mL to prepare your single dose.
- 1 single-dose disposable syringe and needle
- 1 cotton ball or gauze
- 2 alcohol swabs
- 1 sharps disposal container for throwing away (dispose of) your used syringes, needles, and vials. See "How should I dispose of used syringes, needles, and vials?" at the end of this Instructions for Use.
Important:
- Never re-use disposable syringes and needles.
- The vial of mixed INTRON A should be used right away. Do not mix more than 1 vial of INTRON A at a time. If you do not use the vial of prepared solution right away, store it in a refrigerator and use within 24 hours. See the end of this Instructions for Use for information about "How should I store INTRON A?"
- After mixing, throw away (discard) the vial of INTRON A after you withdraw one dose of medicine.
- Make sure you have the right syringe and needle to use with INTRON A. Your healthcare provider should tell you what syringes and needles to use to inject INTRON A.
How should I prepare a dose of INTRON A?
Before you inject INTRON A, the powder must be mixed with 1 mL (cc) of the sterile water for injection (diluent) from the INTRON A vial package.
- 1. Find a clean, well-lit, flat work surface.
- 2. Get one of your INTRON A vial packages. Check the date printed on the carton. Make sure that the expiration date has not passed.
- 3. Wash your hands well with soap and water. Keep your work area, your hands, and injection site clean to decrease the risk of infection (See Figure A).
- 4. Gently warm the vial of diluent by slowly rolling the vial in the palms of your hands for one minute (See Figure B).
- 5. Remove the protective plastic cap from the tops of both vials (INTRON A powder and the diluent). Clean the rubber stopper on the top of both vials with an alcohol swab (See Figure C).
- 6. Open the package for the syringe (See Figure D) you are using and if it does not have a needle attached, then attach a new needle to the syringe.
- 7. Remove the needle cover from the syringe. Fill the syringe with air by pulling the plunger back to 1 mL (See Figure E).
- 8. Hold the diluent vial on your flat work surface. Do not touch the cleaned rubber stopper (See Figure F).
- 9. Push the needle straight down through the middle of the rubber stopper of the diluent vial and slowly inject all the air from the syringe into the air space above the diluent (See Figure G).
- 10.
Keep the needle in the vial. Turn the vial upside down and make sure the tip of the needle is in the diluent.
- Important: The sterile water for injection vial contains an excess amount of sterile water (5 mL). You will only need to withdraw 1 mL to prepare your single dose.
- Slowly pull the plunger back to fill the syringe with diluent to the 1 mL mark on the side of the syringe (See Figure H).
- 11.
With the needle still inserted in the vial, check the syringe for air bubbles (See Figure I).
- If there are any air bubbles, gently tap the syringe with your finger until the air bubbles rise to the top of the syringe.
- Slowly push the plunger up to remove the air bubbles.
- If you push diluent back into the vial, slowly pull back on the plunger to again draw 1 mL of diluent back into the syringe.
- 12. Remove the needle from the vial. Do not let the syringe touch anything.
- 13. Throw away the diluent that is left over in the vial. Do not save any leftover diluent or use it again. See "How should I dispose of used syringes, needles, and vials" at the end of this Instructions for Use.
- 14.
Insert the needle through the center of the rubber stopper of the INTRON A powder vial. Do not touch the cleaned rubber stopper.
- Place the needle tip, at an angle, against the side of the INTRON A powder vial (See Figure J).
- Slowly push the plunger down to inject the diluent into the vial. The stream of liquid should run down the sides of the glass vial.
- To prevent bubbles from forming, do not aim the stream of diluent directly on the medicine in the bottom of the vial.
- Do not remove the needle from the vial.
- 15.
Gently swirl the INTRON A vial in a circular motion until the powder is completely dissolved (See Figure K).
- Do not shake the vial. If any powder remains undissolved in the vial, gently turn the vial upside down until all of the powder is dissolved.
- The solution may look cloudy or bubbly for a few minutes. If air bubbles do form, wait until the solution settles and all bubbles rise to the top. Then withdraw your dose from the vial.
- 16. After the INTRON A completely dissolves, the solution should be clear and colorless to light yellow, without particles. Do not use the mixed solution if you see particles in it, or it is not clear and colorless to light yellow.
- 17.
With the needle in the vial, turn the vial upside down (See Figure L).
- Hold the vial with one hand. Be sure the tip of the needle is in the INTRON A solution. Slowly pull the plunger back to fill the syringe with the exact amount of INTRON A into the syringe that your healthcare provider told you to use.
- 18. With the needle still inserted in the vial, check the syringe for air bubbles. If you see any air bubbles, gently tap the syringe with your finger until the air bubbles rise to the top of the syringe (See Figure M).
- 19. Slowly push the plunger up to remove the air bubbles. If you push solution back into the vial, slowly pull back on the plunger again to draw the correct amount of INTRON A solution back into the syringe.
- 20. Do not remove the needle from the vial. Lay the vial and syringe on its side on your flat work surface until you are ready to inject the INTRON A solution.
How should I choose a site for injection?
Based on your treatment, your healthcare provider will tell you if you should inject a dose of INTRON® A under the skin (subcutaneous injection) or into the muscle (intramuscular injection). If it is too difficult for you to inject, ask someone who has been trained to give injections to help you.
For Subcutaneous Injection
The best sites for injection are areas on your body with a layer of fat between skin and muscle, such as (See Figure N):
- the front of your middle thighs
- the outer area of your upper arms
- the abdomen, except around your belly-button (navel)
For Intramuscular Injection
The best sites for injection into your muscle are (See Figure O):
- the front of your middle thighs
- your upper arms
- the upper outer areas of your buttocks
You should use a different site each time you inject INTRON® A to avoid soreness at any one site. Do not inject INTRON A into an area where the skin is irritated, red, bruised, infected or has scars, stretch marks, or lumps.
How should I inject a dose of INTRON® A?
- 21. Clean the injection site with a new alcohol swab. Wait for the skin to dry.
- 22.
Pick up the vial and syringe from your flat work surface. Remove the syringe and needle from the vial.
- Hold the syringe in the hand that you will use to inject INTRON A.
- Do not touch the needle or allow it to touch the work surface.
- 23. With one hand, pinch a fold of the skin at the cleaned injection site.
- 24.
For subcutaneous injection (under the skin):
- With the other hand, hold the syringe (like a pencil) at a 45-degree angle to the skin. With a quick "dart-like" motion, push the needle into the skin (See Figure P).
- 25.
For intramuscular injection (into the muscle):
- Hold the syringe (like a pencil) at a 90-degree angle to the skin.
- With a quick "dart-like" motion, push the needle into the muscle (See Figure Q).
- 26.
After the needle is inserted, remove the hand used to pinch the skin and use it to hold the syringe barrel.
- Pull the plunger back slightly.
- If no blood is present in the syringe, inject the medicine by gently pushing the plunger all the way down the syringe barrel, until the syringe is empty.
-
If blood comes into the syringe, the needle has entered a blood vessel. Do not inject INTRON® A.
- Withdraw the needle and dispose of the syringe and needle in the sharps disposal container. See section "How should I dispose of used syringes, needles, and vials?" at the end of this Instructions for Use.
- If there is bleeding, cover the injection site with a bandage.
- Then, repeat steps 1 through 25 with a new dose of INTRON A and inject the medicine at a new injection site.
- 27.
When the syringe is empty, pull the needle out of the skin.
- Place a cotton ball or gauze over the injection site and press for several seconds. Do not massage the injection site.
- If there is bleeding, cover the injection site with a bandage.
- 28. Dispose of used syringes, needles, and vials in the sharps disposal container. (See "How should I dispose of used syringes, needles, and vials?" below.)
How should I dispose of used syringes, needles, and vials?
- Put your used needles, syringes and vials in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles, syringes and vials in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used syringes and needles. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Always keep the sharps disposal container out of the reach of children.
- Before mixing, store in the refrigerator between 36°F to 46°F (2°C to 8°C).
- After mixing the INTRON A Powder for Injection, use the solution right away or store the solution in the refrigerator for up to 24 hours between 36°F to 46°F (2°C to 8°C). Allow the solution to come to room temperature before using.
- Do not freeze Intron A.
- Keep away from heat.
- Throw away any medicine left in the vial after you withdraw 1 dose.
Keep INTRON A and all medicines out of the reach of children.
-
SPL UNCLASSIFIED SECTION
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
U.S. License Number 0002For patent information: www.merck.com/product/patent/home.html
Copyright © 1996- 2019, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.Revised: 08/2019
usifu-mk2958-pwi-5ml-1908r002 -
PRINCIPAL DISPLAY PANEL - Kit Carton - 10 Million IU
NDC 0085-4350-01
RefrigerateInterferon alfa-2b,
recombinantIntron® A
Powder for Injection
Single-Use Vial10 million IU per vial
For Intramuscular/Subcutaneous/Intravenous/
Intralesional Use.Rx only
Medication Guide for patient enclosed.
Dosage and Administration:
See package insert.
Reconstitute as directed in package insert.
Read accompanying directions.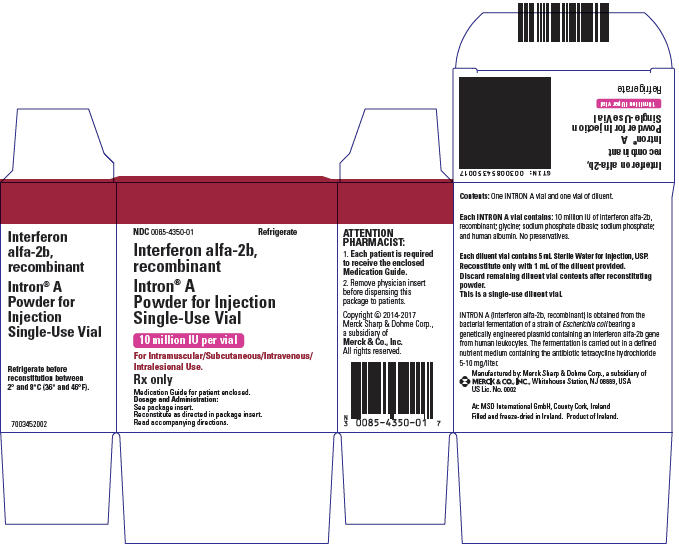
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 18 Million IU
NDC 0085-4351-01
Refrigerate(Interferon alfa-2b, recombinant)
Intron® A
Powder for Injection
Single-Use Vial18 million IU per vial
For Intramuscular/Subcutaneous/
Intravenous Use
NOT FOR INTRALESIONAL USE.INTRON® A
Interferon Alfa-2b, Recombinant
for InjectionRx only
Medication Guide for patient enclosed.
Dosage and Administration: See package insert.
Read accompanying directions.
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 50 Million IU
NDC 0085-4352-01
Refrigerate(Interferon alfa-2b, recombinant)
Intron® A
Powder for Injection
Single-Use Vial50 million IU per vial
For Intramuscular/Subcutaneous/
Intravenous Use
NOT FOR INTRALESIONAL USE.INTRON® A
Interferon Alfa-2b, Recombinant
for InjectionRx only
Medication Guide for patient enclosed.
Dosage and Administration: See package insert.
Read accompanying directions.
-
PRINCIPAL DISPLAY PANEL - 18 Million IU Vial Carton
NDC 0085-1168-01
Refrigerate(Interferon alfa-2b, recombinant)
Intron® A
Solution for Injection18 million IU multidose vial
3 million IU/0.5 mLFor Intramuscular/Subcutaneous Use.
NOT FOR INTRALESIONAL USE.INTRON® A
Interferon Alfa-2b, Recombinant
for InjectionRx only
Medication Guide for patient enclosed.
Dosage and Administration: See package insert.
Read accompanying directions.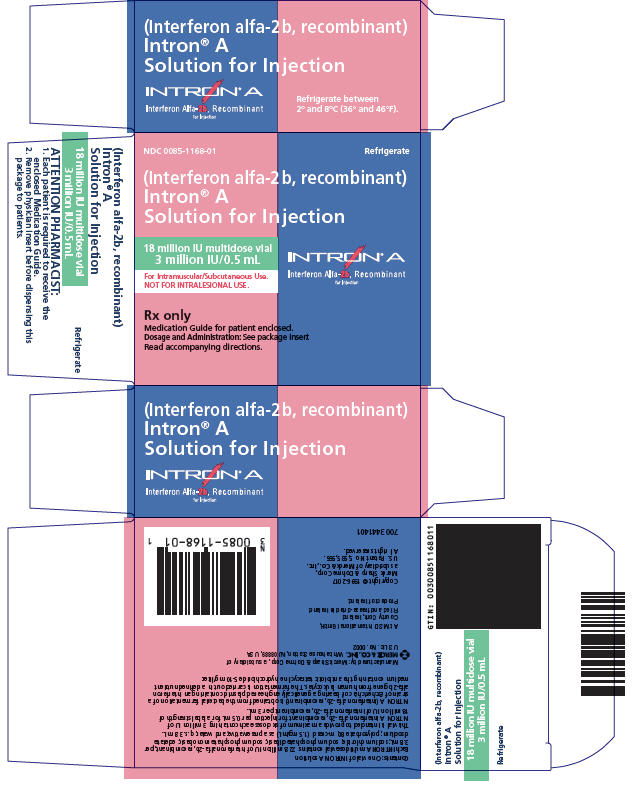
-
PRINCIPAL DISPLAY PANEL - 25 Million IU Vial Carton
NDC 0085-1133-01
Refrigerate(Interferon alfa-2b, recombinant)
Intron® A
Solution for Injection25 million IU multidose vial
5 million IU/0.5 mLFor Intramuscular/Subcutaneous/
Intralesional UseINTRON® A
Interferon Alfa-2b, Recombinant
for InjectionRx only
Medication Guide for patient enclosed.
Dosage and Administration: See package insert.
Read accompanying directions.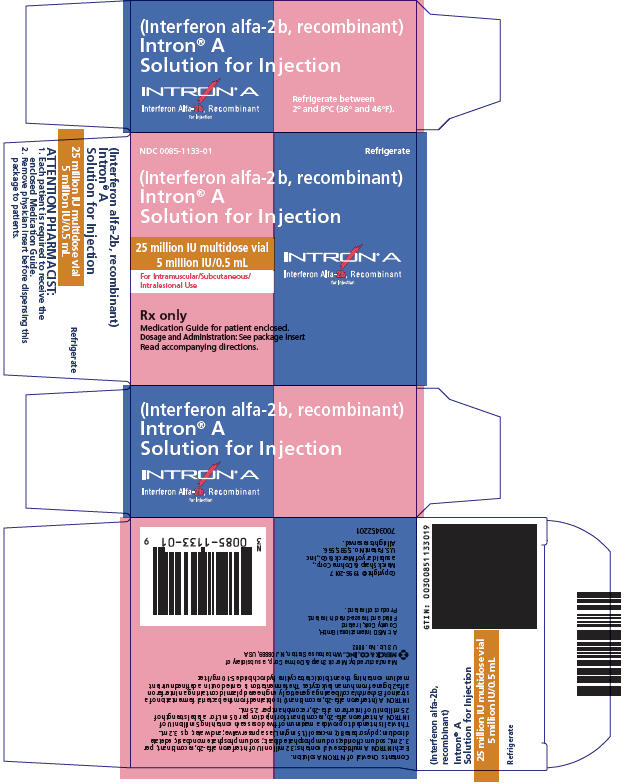
-
INGREDIENTS AND APPEARANCE
INTRON A
interferon alfa-2b kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0085-4350 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0085-4350-01 1 in 1 CARTON 08/11/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1.25 mL Part 2 1 VIAL 5 mL Part 1 of 2 INTRON A
interferon alfa-2b injection, powder, for solutionProduct Information Route of Administration INTRAMUSCULAR, SUBCUTANEOUS, INTRALESIONAL, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength interferon alfa-2b (UNII: 43K1W2T1M6) (interferon alfa-2b - UNII:43K1W2T1M6) interferon alfa-2b 38 ug in 1 mL Inactive Ingredients Ingredient Name Strength Glycine (UNII: TE7660XO1C) 20 mg in 1 mL SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) 2.3 mg in 1 mL Sodium Phosphate, Monobasic, Unspecified form (UNII: 3980JIH2SW) 0.55 mg in 1 mL Albumin Human (UNII: ZIF514RVZR) 1 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1.25 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103132 06/04/1986 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC: 0085-4346 Route of Administration INTRAMUSCULAR, SUBCUTANEOUS, INTRALESIONAL, INTRAVENOUS Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0085-4346-01 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103132 08/11/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103132 08/11/2014 INTRON A
interferon alfa-2b kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0085-4351 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0085-4351-01 1 in 1 CARTON 08/11/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1.25 mL Part 2 1 VIAL 5 mL Part 1 of 2 INTRON A
interferon alfa-2b injection, powder, for solutionProduct Information Route of Administration INTRAMUSCULAR, SUBCUTANEOUS, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength interferon alfa-2b (UNII: 43K1W2T1M6) (interferon alfa-2b - UNII:43K1W2T1M6) interferon alfa-2b 69 ug in 1 mL Inactive Ingredients Ingredient Name Strength Glycine (UNII: TE7660XO1C) 20 mg in 1 mL SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) 2.3 mg in 1 mL Sodium Phosphate, Monobasic, Unspecified form (UNII: 3980JIH2SW) 0.55 mg in 1 mL Albumin Human (UNII: ZIF514RVZR) 1 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1.25 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103132 06/04/1986 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC: 0085-4346 Route of Administration INTRAMUSCULAR, SUBCUTANEOUS, INTRAVENOUS Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0085-4346-01 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103132 08/11/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103132 08/11/2014 INTRON A
interferon alfa-2b kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0085-4352 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0085-4352-01 1 in 1 CARTON 08/11/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1.25 mL Part 2 1 VIAL 5 mL Part 1 of 2 INTRON A
interferon alfa-2b injection, powder, for solutionProduct Information Route of Administration INTRAMUSCULAR, SUBCUTANEOUS, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength interferon alfa-2b (UNII: 43K1W2T1M6) (interferon alfa-2b - UNII:43K1W2T1M6) interferon alfa-2b 192 ug in 1 mL Inactive Ingredients Ingredient Name Strength Glycine (UNII: TE7660XO1C) 20 mg in 1 mL SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) 2.3 mg in 1 mL Sodium Phosphate, Monobasic, Unspecified form (UNII: 3980JIH2SW) 0.55 mg in 1 mL Albumin Human (UNII: ZIF514RVZR) 1 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1.25 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103132 06/04/1986 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC: 0085-4346 Route of Administration INTRAMUSCULAR, SUBCUTANEOUS, INTRAVENOUS Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0085-4346-01 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103132 08/11/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103132 08/11/2014 INTRON A
interferon alfa-2b injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0085-1168 Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Interferon alfa-2b (UNII: 43K1W2T1M6) (Interferon alfa-2b - UNII:43K1W2T1M6) Interferon alfa-2b 11.6 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) 3.75 mg in 0.5 mL SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) 0.9 mg in 0.5 mL Sodium Phosphate, Monobasic, Unspecified form (UNII: 3980JIH2SW) 0.65 mg in 0.5 mL edetate disodium (UNII: 7FLD91C86K) 0.05 mg in 0.5 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.05 mg in 0.5 mL metacresol (UNII: GGO4Y809LO) 0.75 mg in 0.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0085-1168-01 1 in 1 CARTON 06/04/1986 1 3.8 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103132 06/04/1986 INTRON A
interferon alfa-2b injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0085-1133 Route of Administration SUBCUTANEOUS, INTRAMUSCULAR, INTRALESIONAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Interferon alfa-2b (UNII: 43K1W2T1M6) (Interferon alfa-2b - UNII:43K1W2T1M6) Interferon alfa-2b 19.2 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) 3.75 mg in 0.5 mL SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) 0.9 mg in 0.5 mL Sodium Phosphate, Monobasic, Unspecified form (UNII: 3980JIH2SW) 0.65 mg in 0.5 mL edetate disodium (UNII: 7FLD91C86K) 0.05 mg in 0.5 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.05 mg in 0.5 mL metacresol (UNII: GGO4Y809LO) 0.75 mg in 0.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0085-1133-01 1 in 1 CARTON 06/04/1986 1 3.2 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103132 06/04/1986 Labeler - Merck Sharp & Dohme Corp. (001317601)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.