EQUILIBRIUM CBD Pain Relief Balm
EQUILIBRIUM CBD Pain Relief Balm by
Drug Labeling and Warnings
EQUILIBRIUM CBD Pain Relief Balm by is a Otc medication manufactured, distributed, or labeled by EQUILIBRIUM NUTRITION PRODUCTS, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
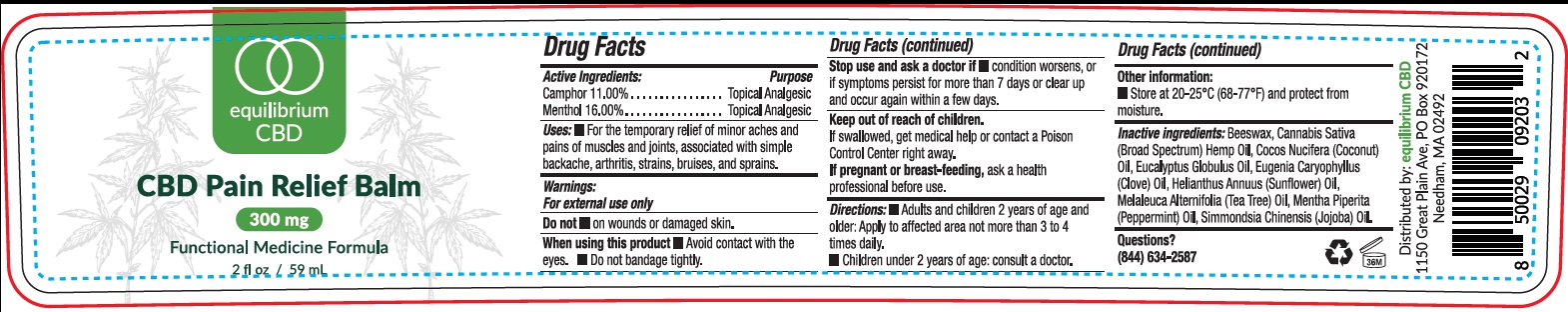
EQUILIBRIUM CBD PAIN RELIEF BALM- camphor (synthetic), menthol cream
EQUILIBRIUM NUTRITION PRODUCTS, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
EQUILIBRIUM CBD Pain Relief Balm
Uses:
- For the temporary relief of minor aches and pains of muscles and joints, associated with simple backache, arthritis, strains, bruises, and sprains.
Warnings:
For external use only
Stop use and ask a doctor if
- condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days.
Directions:
- Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily.
- Children under 2 years of age: consult a doctor.
Inactive ingredients:
Beeswax, Cannabis Sativa (Broad Spectrum) Hemp Oil, Cocos Nucifera (Coconut) Oil, Eucalyptus Globulus Oil, Eugenia Caryophyllus (Clove) Oil, Helianthus Annuus (Sunflower) Oil, Melaleuca Alternifolia (Tea Tree) Oil, Mentha Piperita (Peppermint) Oil, Simmondsia Chinensis (Jojoba) Oil
| EQUILIBRIUM CBD PAIN RELIEF BALM
camphor (synthetic), menthol cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - EQUILIBRIUM NUTRITION PRODUCTS, LLC (056364241) |