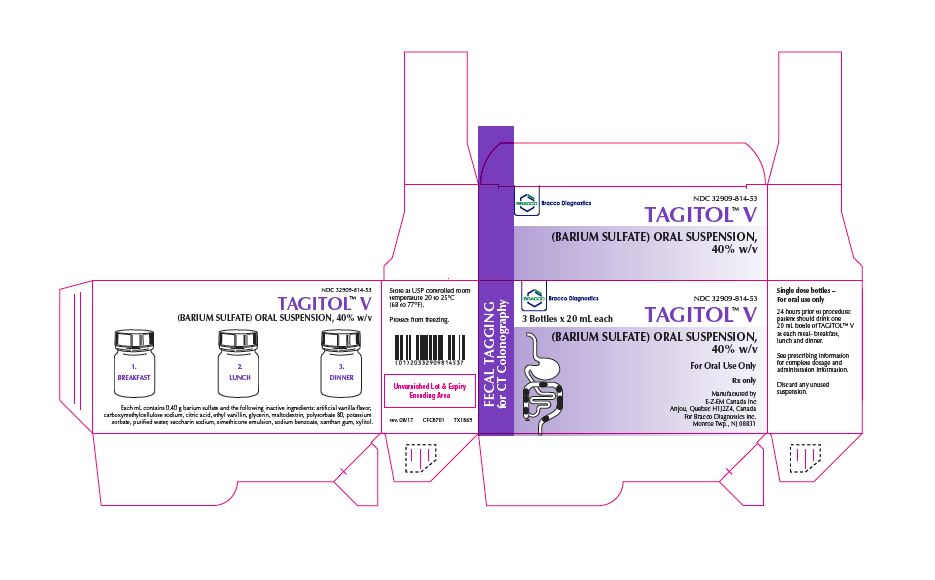
TAGITOL V- barium sulfate suspension
Tagitol V by
Drug Labeling and Warnings
Tagitol V by is a Prescription medication manufactured, distributed, or labeled by E-Z-EM Canada Inc, E-Z-EM, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TAGITOL V safely and effectively. See full prescribing information for TAGITOL V.
TAGITOL V (barium sulfate) oral suspension
Initial U.S. Approval: 2016INDICATIONS AND USAGE
TAGITOL V is a radiographic contrast agent indicated in adult patients for use in computed tomography (CT) colonography as a fecal tagging agent (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Oral suspension : barium sulfate (40% w/v) 20 mL single dose bottles as a ready to use suspension for oral administration (3)
CONTRAINDICATIONS
TAGITOL V is contraindicated in patients with:
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions: Emergency equipment and trained personnel should be immediately available (5.1)
- Intra-abdominal barium leakage: May occur in conditions which increase the risk of perforation such as - carcinoma, GI fistula, inflammatory bowel disease, gastric or duodenal ulcer, appendicitis, diverticulitis, or severe stenosis or obstructing lesions of the GI tract (5.2)
- Delayed GI transit and obstruction: Patients should maintain adequate hydration in days following a barium sulfate procedure to avoid obstruction or impaction by baroliths (5.3)
- Aspiration pneumonitis: Caution is recommended in patients with a history of food aspiration and in patients with known swallowing disorders (5.4)
ADVERSE REACTIONS
Common adverse reactions include nausea, vomiting, diarrhea and abdominal cramping (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bracco Diagnostics Inc at 1-800-257-5181 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Intra-abdominal Barium Leakage
5.3 Delayed Gastrointestinal Transit and Obstruction
5.4 Aspiration Pneumonitis
5.5 Systemic Embolization
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
- The recommended oral dose of TAGITOL V is one 20 mL bottle (8 g barium sulfate) with each meal (breakfast, lunch and dinner) the day before the colonography examination. Total dose = 3 bottles (24 g barium sulfate).
2.2 Important Administration Instructions
- TAGITOL V is typically provided to the patient for self-administration. Advise patients to carefully read and follow the Patient Instructions for Use to be provided to the patient.
- Shake bottle for 15 seconds prior to administration.
- For oral use only.
- Encourage patients to hydrate following the barium sulfate procedure.
- Discard any unused suspension.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
TAGITOL V is contraindicated in patients with:
- known or suspected perforation of the gastrointestinal (GI) tract;
- known obstruction of the GI tract;
- high risk of GI perforation such as those with a recent GI perforation, acute GI hemorrhage or ischemia, toxic megacolon, severe ileus, post GI surgery or biopsy, acute GI injury or burn, or recent radiotherapy to the pelvis;
- high risk of aspiration such as those with prior aspiration, tracheo-esophageal fistula, or obtundation;
- known hypersensitivity to barium sulfate or any of the excipients of TAGITOL V.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Barium sulfate preparations contain a number of excipients, including natural and artificial flavors and may induce serious hypersensitivity reactions. The manifestations include hypotension, bronchospasm and other respiratory impairments, dermal reactions including rashes, urticaria and itching. A history of bronchial asthma, atopy, or a previous reaction to a contrast agent may increase the risk for hypersensitivity reactions. Emergency equipment and trained personnel should be immediately available for treatment of a hypersensitivity reaction.
5.2 Intra-abdominal Barium Leakage
The use of TAGITOL V is contraindicated in patients at high risk of perforation of the GI tract [see Contraindications (4)]. Administration of TAGITOL V may result in leakage of barium from the GI tract in the presence of conditions that increase the risk of perforation such as carcinomas, GI fistula, inflammatory bowel disease, gastric or duodenal ulcer, appendicitis, or diverticulitis, and in patients with a severe stenosis at any level of the gastrointestinal tract, especially if it is distal to the stomach. The barium leakage has been associated with peritonitis and granuloma formation.
5.3 Delayed Gastrointestinal Transit and Obstruction
Orally administered barium sulfate may accumulate proximal to a constricting lesion of the colon, causing obstruction or impaction with development of baroliths (inspissated barium associated with feces) and may lead to abdominal pain, appendicitis, bowel obstruction, or rarely perforation. Patients with the following conditions are at higher risk for developing obstruction or baroliths: severe stenosis at any level of the GI tract, impaired gastrointestinal motility, electrolyte imbalance, dehydration, on a low residue diet, on medications that delay GI motility, constipation, cystic fibrosis, Hirschsprung disease, and the elderly. To reduce the risk of delayed GI transit and obstruction, patients should maintain adequate hydration following a barium sulfate procedure.
5.4 Aspiration Pneumonitis
The use of TAGITOL V is contraindicated in patients at high risk of aspiration [see Contraindications (4)]. Oral administration of barium is associated with aspiration pneumonitis, especially in patients with a history of food aspiration or with compromised swallowing mechanism. Vomiting following oral administration of barium sulfate may lead to aspiration pneumonitis.
5.5 Systemic Embolization
Barium sulfate products may occasionally intravasate into the venous drainage of the large bowel and enter the circulation as a "barium embolus" leading to potentially fatal complications which include systemic and pulmonary embolism, disseminated intravascular coagulation, septicemia and prolonged severe hypotension. Although this complication is exceedingly uncommon after oral administration of barium sulfate products, monitor patients for potential intravasation when administering barium sulfate.
-
6 ADVERSE REACTIONS
The following adverse reactions have been identified from spontaneous reporting or clinical studies of barium sulfate administered orally. Because the reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to drug exposure:
- Nausea, vomiting, diarrhea and abdominal cramping
- Serious adverse reactions and fatalities include aspiration pneumonitis, barium sulfate impaction, intestinal perforation with consequent peritonitis and granuloma formation, vasovagal and syncopal episodes.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
TAGITOLVis not absorbed systemically following oral administration, and maternal use is not expected to result in fetal exposure to the drug [see Clinical Pharmacology (12.3)].
8.2 Lactation
TAGITOL V is not absorbed systemically by the mother following oral administration and breastfeeding is not expected to result in exposure of the infant to the drug [see Clinical Pharmacology (12.3)]
8.5 Geriatric Use
Clinical studies of TAGITOL V do not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
11 DESCRIPTION
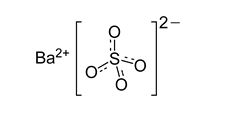
TAGITOL V (barium sulfate) is a radiographic contrast agent that is supplied as a 40% w/v, off-white to lightly colored, free-flowing, ready-to-use suspension with an apple aroma for oral administration. The active ingredient barium sulfate is designated chemically as BaSO4 with a molecular weight of 233.4 g/mol and the following chemical structure:
TAGITOL V contains the following excipients: carboxymethylcellulose sodium, citric acid, glycerin, maltodextrin, natural and artificial apple flavor, polysorbate 80, potassium sorbate, purified water, saccharin sodium, simethicone emulsion, sodium benzoate, sodium citrate, xanthan gum, and xylitol.
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW
SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
TAGITOL V (barium sulfate) is an oral suspension (40% w/v) supplied as a box of three 20 mL HDPE bottles. Each bottle contains 8 grams barium sulfate.
Provided as: 24 boxes, each containing 3 (20 mL) bottles (NDC: 32909-814)
-
17 PATIENT COUNSELING INFORMATION
After administration, advise patients to:
- Maintain adequate hydration [see Dosage and Administration (2.2) and Warnings and Precautions (5.3)].
- Seek medical attention for worsening of constipation or slow gastrointestinal passage [see Warnings and Precautions (5.3)].
- Seek medical attention for any delayed onset of hypersensitivity: rash, urticaria, or respiratory difficulty [see Warnings and Precautions (5.1)].
TAGITOL V is typically provided to the patient for self-administration. Advise patients to carefully read and follow the Patient Instructions for Use to be provided to the patient.
Provide the patient with any site specific instructions regarding their procedure and when to take meals.
Manufactured by
EZEM Canada Inc
Anjou (Quebec) Canada H1J 2Z4
For
Bracco Diagnostics Inc.
Monroe Township, NJ 08831 -
INSTRUCTIONS FOR USE
Read this Instructions for Use before you drink TAGITOL V (barium sulfate) oral suspension. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Take TAGITOL V exactly as your healthcare provider tells you. Your healthcare provider will prescribe the dose that is right for you. You can ask your healthcare provider or pharmacist if you have any questions about how to take TAGITOL V.
- Before using TAGITOL V store at room temperature between 68°F and 77°F (20°C and 25°C).
- Do not freeze.
Keep TAGITOL V and all medicines out of the reach of children.
- 1 box containing 3 bottles of TAGITOL V. Each bottle contains 20 mL of TAGITOL V
The day before your procedure you will drink 1 bottle of TAGITOL V with each meal:
- Breakfast: Shake 1 bottle of TAGITOL V for 15 seconds, open the bottle, and drink the liquid with breakfast.
- Lunch: Shake 1 bottle of TAGITOL V for 15 seconds, open the bottle, and drink the liquid with lunch.
- Dinner: Shake 1 bottle of TAGITOL V for 15 seconds, open the bottle, and drink the liquid with dinner.
Throw away any unused TAGITOL V with normal household trash. Do not throw away by flushing down the drain.
What should I do if the TAGITOL V spills?
If you spill the liquid while shaking or drinking it, you can clean it up. TAGITOL V is not harmful and can be thrown away with normal household trash.
If you spilled any of the liquid, check with your healthcare provider to find out if you need to change the date of the appointment for your procedure.
This Instructions for Use has been approved by the U.S. Food and Drug Administration
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TAGITOL V
barium sulfate suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 32909-814 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Barium Sulfate (UNII: 25BB7EKE2E) (Barium Sulfate - UNII:25BB7EKE2E) Barium Sulfate 400 mg in 1 mL Inactive Ingredients Ingredient Name Strength anhydrous citric acid (UNII: XF417D3PSL) dimethicone 350 (UNII: 2Y53S6ATLU) dimethicone 1000 (UNII: MCU2324216) glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) polysorbate 80 (UNII: 6OZP39ZG8H) potassium sorbate (UNII: 1VPU26JZZ4) saccharin sodium (UNII: SB8ZUX40TY) silicon dioxide (UNII: ETJ7Z6XBU4) sodium benzoate (UNII: OJ245FE5EU) trisodium citrate dihydrate (UNII: B22547B95K) water (UNII: 059QF0KO0R) xanthan gum (UNII: TTV12P4NEE) xylitol (UNII: VCQ006KQ1E) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) Product Characteristics Color WHITE Score Shape Size Flavor APPLE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 32909-814-53 24 in 1 CASE 08/04/2017 1 3 in 1 BOX 1 20 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208143 08/04/2017 Labeler - E-Z-EM Canada Inc (204211163) Registrant - E-Z-EM, INC. (002041226)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.