Hangover Patch, Vitamin Patch
Hangover Patch, Vitamin Patch by
Drug Labeling and Warnings
Hangover Patch, Vitamin Patch by is a Otc medication manufactured, distributed, or labeled by ZheJiang Longmed Medical Technology Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HANGOVER PATCH, VITAMIN PATCH- vitamin c patch
ZheJiang Longmed Medical Technology Co., Ltd.
----------
Hangover Patch, Vitamin Patch
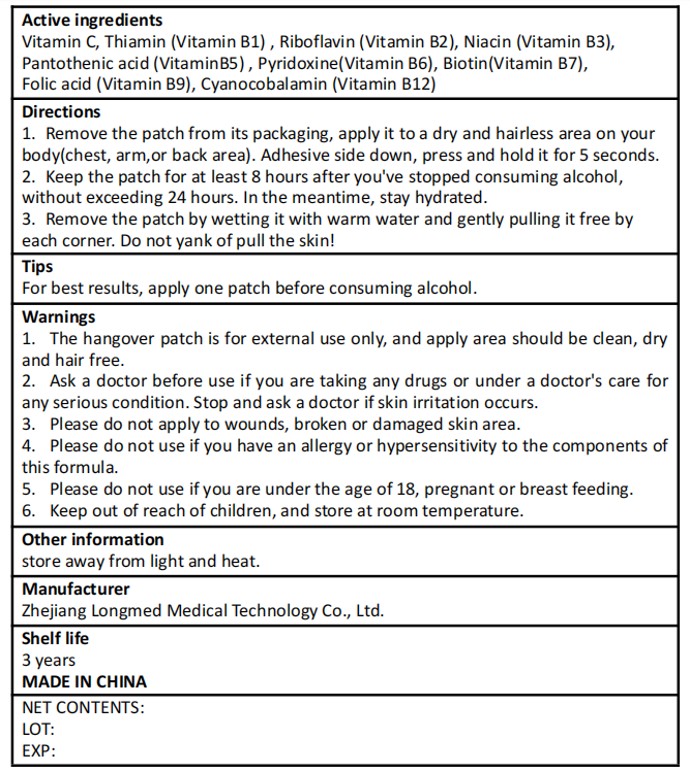
Vitamin C
Thiamin (Vitamin B1)
Riboflavin (Vitamin B2)
Niacin (Vitamin B3)
Pantothenic acid (VitaminB5)
Pyridoxine(Vitamin B6)
Biotin(Vitamin B7)
Folic acid (Vitamin B9)
Cyanocobalamin (Vitamin B12)
1. The hangover patch is for external use only, and apply area should be clean, dryand hair free.
2. Ask a doctor before use if you are taking any drugs or under a doctor's care forany serious condition.Stop and ask a doctor if skin irritation occurs.3. Please do not apply to wounds, broken or damaged skin area.4. Please do not use if you have an allergy or hypersensitivity to the components ofthis formula.
5. Please do not use if you are under the age of 18, pregnant or breast feeding.
Keep out of reach of children, and store at room temperature.
1. Remove the patch from its packaging, apply it to a dry and hairless area on yourbody(chest, arm,or back area). Adhesive side down, press and hold it for 5 seconds.
2. Keep the patch for at least 8 hours after you've stopped consuming alcohol,without exceeding 24 hours. In the meantime, stay hydrated.
3. Remove the patch by wetting it with warm water and gently pulling it free byeach corner. Do not yank of pull the skin!
| HANGOVER PATCH, VITAMIN PATCH
vitamin c patch |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Labeler - ZheJiang Longmed Medical Technology Co., Ltd. (554468373) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ZheJiang Longmed Medical Technology Co., Ltd. | 554468373 | manufacture(84534-004) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.