ACTIVATED MINERAL SUNSCREEN TINTED cream
Activated Mineral Sunscreen Tinted by
Drug Labeling and Warnings
Activated Mineral Sunscreen Tinted by is a Otc medication manufactured, distributed, or labeled by Reve Skincare LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Uses
- Warnings
-
Directions
apply generously 15 minutes before sun exposure
reapply: at least every 2 hours.use a water resistant sunscreen if swimming or sweating
Sun Protection Measures. Spending time in the sunincreases your risk of skin cancer and early skin aging. To decrease
this risk, regularly use a sunscreen with a Broad Spectrum SPF
value of 15 or higher and other sun protection measures including:
limit time in the sun, especially from 10 a.m. - 2 p.m.
wear long-sleeved shirts, pants, hats, and sunglasses
children under 6 months of age: Ask a doctor -
Inactive ingredients
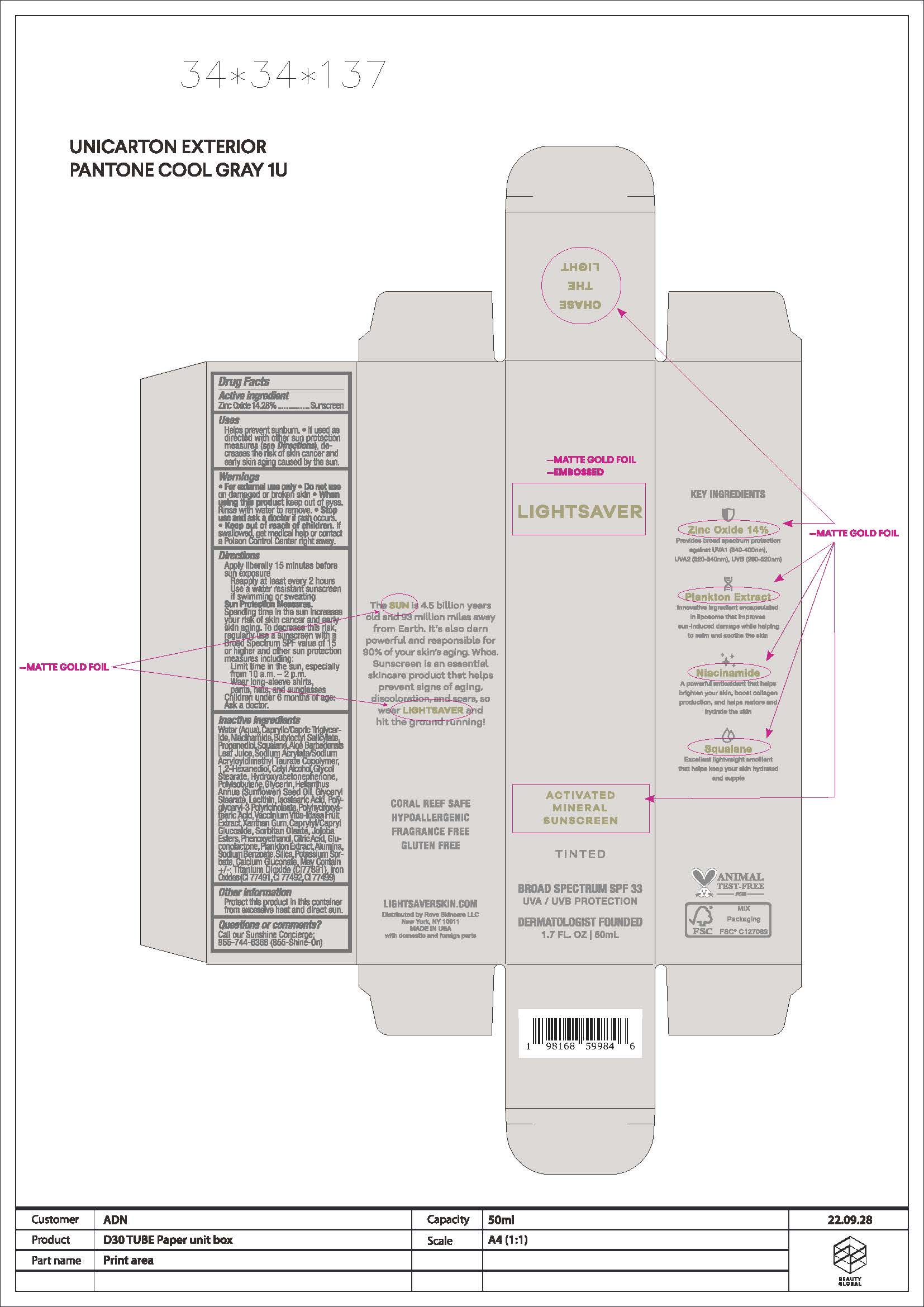
Water, Caprylic/Capric Triglyceride, Niacinamide, Butyloctyl Salicylate, Propanediol, Squalene, Aloe Barbadensis Leaf Juice, Sodium Acrylate/Sodium Acyloyldimethyl Tauarate Copolymer, 1,2-Hexanediol, Cetyl Alcohol, Glycol Stearate, Helianthus Annuus (Sunflower) Seed Oil, Hydroxyacetophenone, Polyisobutene, Glycerin, Glyceryl Stearate, Lecithin, Isostearic Acid, Polyglyceryl-3 Polyricinoleate, Polyhydroxystearic Acid, Vaccinium Vitis-Idaea Fruit Extract, Xanthan Gum, Caprylyl/Capryl Glucoside, Sorbitan Oleate, Phenoxyethanol, Jojoba Esters, Citric Acid, Gluconolactone, Plankotn Extract, Alumina, Sodium Benzoate, Potassium Sorbate, Silica, Calcium Glucate. May Contain (+/-): Titanium Dioxide (CI77891), Iron Oxides (CI 774941, 77492, 77499)
- Other Information
- Questions or Comments?
- Purpose
- Keep out of reach of children.
- Topical
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ACTIVATED MINERAL SUNSCREEN TINTED
activated mineral sunscreen tinted creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 83083-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 142.8 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PROPANEDIOL (UNII: 5965N8W85T) SQUALANE (UNII: GW89575KF9) ALOE VERA LEAF (UNII: ZY81Z83H0X) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCOL STEARATE (UNII: 0324G66D0E) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) GLYCERIN (UNII: PDC6A3C0OX) HELIANTHUS ANNUUS WHOLE (UNII: 17S27ZT6KR) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) VACCINIUM VITIS-IDAEA FRUIT OIL (UNII: 16Y54799WZ) ISOHEXADECANE (UNII: 918X1OUF1E) XANTHAN GUM (UNII: TTV12P4NEE) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PEG-6 SORBITAN OLEATE (UNII: 58O7V09UCI) PHENOXYETHANOL (UNII: HIE492ZZ3T) JOJOBA OIL (UNII: 724GKU717M) CITRIC ACID ACETATE (UNII: DSO12WL7AU) GLUCONOLACTONE (UNII: WQ29KQ9POT) ALUMINUM (UNII: CPD4NFA903) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SILICON (UNII: Z4152N8IUI) CALCIUM GLUCONATE (UNII: SQE6VB453K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BROWN IRON OXIDE (UNII: 1N032N7MFO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83083-002-50 1 in 1 CARTON 03/25/2025 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/25/2025 Labeler - Reve Skincare LLC (118831764)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.