NIOXIN PRO CLINICAL SCALP RECOVERY PURIFYING- pyrithione zinc shampoo
Nioxin PRO CLINICAL SCALP RECOVERY purifying by
Drug Labeling and Warnings
Nioxin PRO CLINICAL SCALP RECOVERY purifying by is a Otc medication manufactured, distributed, or labeled by Wella Operations US LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
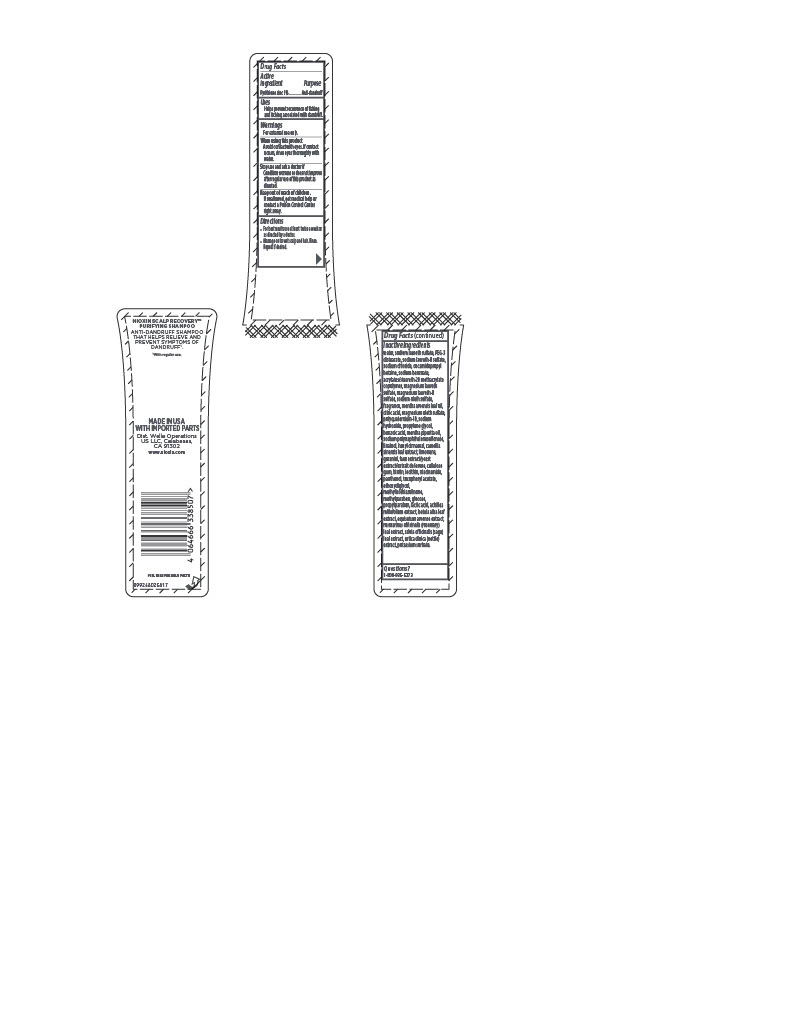
Inactive ingredients water, sodium laureth sulfate, PEG-3 distearate, sodium laureth-8 sulfate, sodium chloride, cocamidopropyl betaine, sodium benzoate, acrylates/steareth-20 methacrylate copolymer, magnesium laureth sulfate, magnesium laureth-8 sulfate, sodium oleth sulfate, fragrance, mentha arvensis leaf oil, citric acid, magnesium oleth sulfate, polyquaternium-10, sodium hydroxide, propylene glycol, benzoic acid, mentha piperita oil, sodium polynaphthalenesulfonate, linalool, hexyl cinnamal, camellia sinensis leaf extract, limonene, geraniol, faex extract/yeast extract/extrait de levure, cellulose gum, biotin, lecithin, niacinamide, panthenol, tocopheryl acetate, ethoxydiglycol, methylisothiazolinone, methylparaben, glucose, propylparaben, lactic acid, achillea millefolium extract, betula alba leaf extract, equisetum arvense extract, rosmarinus officinalis (rosemary) leaf extract, salvia officinalis (sage) leaf extract, urtica dioica (nettle) extract, potassium sorbate.
- SPL UNCLASSIFIED SECTION
- QUESTIONS
-
PRINCIPAL DISPLAY PANEL
nioxin®
PRO CLINICAL
SCALP RECOVERY™
purifying shampoo
FOR ANTI-DANDRUFF
FOR ITCHY, FLAKY SCALP COMBATS DANDRUFF FROM THE 1 ST USE WITH PYRITHIONE ZINC.
CLINICALLY & DERMATOLOGICALLY TESTED
200 mL | 6.7 FL OZ

nioxin®
PRO CLINICAL
SCALP RECOVERY™
purifying shampoo
FOR ANTI-DANDRUFF
FOR ITCHY, FLAKY SCALP COMBATS DANDRUFF FROM THE 1 ST USE WITH PYRITHIONE ZINC.
CLINICALLY & DERMATOLOGICALLY TESTED
1L | 33.8 FL OZ


-
INGREDIENTS AND APPEARANCE
NIOXIN PRO CLINICAL SCALP RECOVERY PURIFYING
pyrithione zinc shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 82157-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 1 g in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM LAURETH-8 SULFATE (UNII: YP8U3694P0) MENTHA ARVENSIS LEAF OIL (UNII: 1AEY1M553N) CITRIC ACID (UNII: 2968PHW8QP) MENTHA PIPERITA (PEPPERMINT) OIL (UNII: AV092KU4JH) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) METHYLPARABEN (UNII: A2I8C7HI9T) BETULA PUBESCENS LEAF (UNII: 84SOH0O3OO) HEXYL CINNAMAL (UNII: 7X6O37OK2I) WATER (UNII: 059QF0KO0R) POLYQUATERNIUM-10 (400 MPA.S AT 2%) (UNII: HB1401PQFS) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) ETHOXYDIGLYCOL (UNII: A1A1I8X02B) ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) BIOTIN (UNII: 6SO6U10H04) SODIUM HYDROXIDE (UNII: 55X04QC32I) FORMALDEHYDE/SODIUM NAPHTHALENESULFONATE COPOLYMER (3000 MW) (UNII: 90D834OZUI) LINALOOL, (+/-)- (UNII: D81QY6I88E) CAMELLIA SINENSIS LEAF (UNII: W2ZU1RY8B0) HEXAMETHYLINDANOPYRAN (UNII: 14170060AT) GERANIOL (UNII: L837108USY) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PROPYLPARABEN (UNII: Z8IX2SC1OH) ROSEMARY (UNII: IJ67X351P9) SAGE (UNII: 065C5D077J) URTICA DIOICA LEAF (UNII: X6M0DRN46Q) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CALCIUM PANTOTHENATE (UNII: 568ET80C3D) SODIUM CHLORIDE (UNII: 451W47IQ8X) INOSITOL (UNII: 4L6452S749) CELLULOSE GUM (UNII: K679OBS311) NIACINAMIDE (UNII: 25X51I8RD4) EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) PANTHENOL (UNII: WV9CM0O67Z) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) MAGNESIUM LAURETH SULFATE (UNII: UKW9G007TZ) SODIUM OLETH SULFATE (UNII: 3MX3FTR4XP) CARAMEL (UNII: T9D99G2B1R) TARTARIC ACID (UNII: W4888I119H) MAGNESIUM LAURETH-8 SULFATE (UNII: 2OTJ9LF5UA) ACRYLATES/STEARETH-20 METHACRYLATE COPOLYMER (UNII: EPA1872R1N) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BENZOIC ACID (UNII: 8SKN0B0MIM) PEG-3 DISTEARATE (UNII: 8420ECX438) SODIUM BENZOATE (UNII: OJ245FE5EU) LACTIC ACID (UNII: 33X04XA5AT) LIMONENE, (+/-)- (UNII: 9MC3I34447) MAGNESIUM OLETH SULFATE (UNII: LCQ4WP3DTL) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) GLUCOSE (UNII: 5SL0G7R0OK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82157-011-10 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/01/2025 2 NDC: 82157-011-20 200 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/01/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 04/01/2025 Labeler - Wella Operations US LLC (117781338)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.