ZEMAIRA- alpha-1-proteinase inhibitor human kit kit
ZEMAIRA by
Drug Labeling and Warnings
ZEMAIRA by is a Other medication manufactured, distributed, or labeled by Fisher Clinical Services Inc., Fisher Clinical Services. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
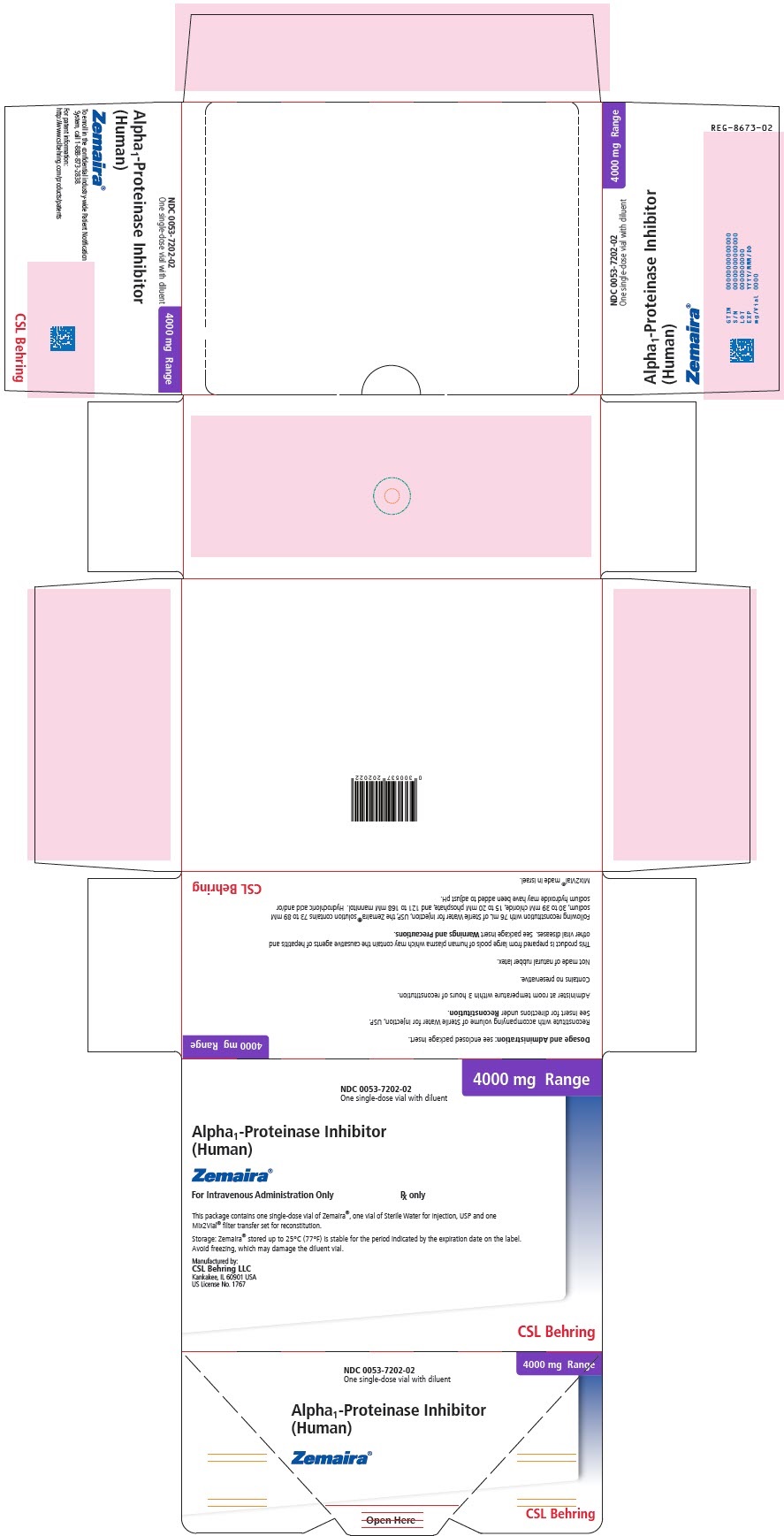
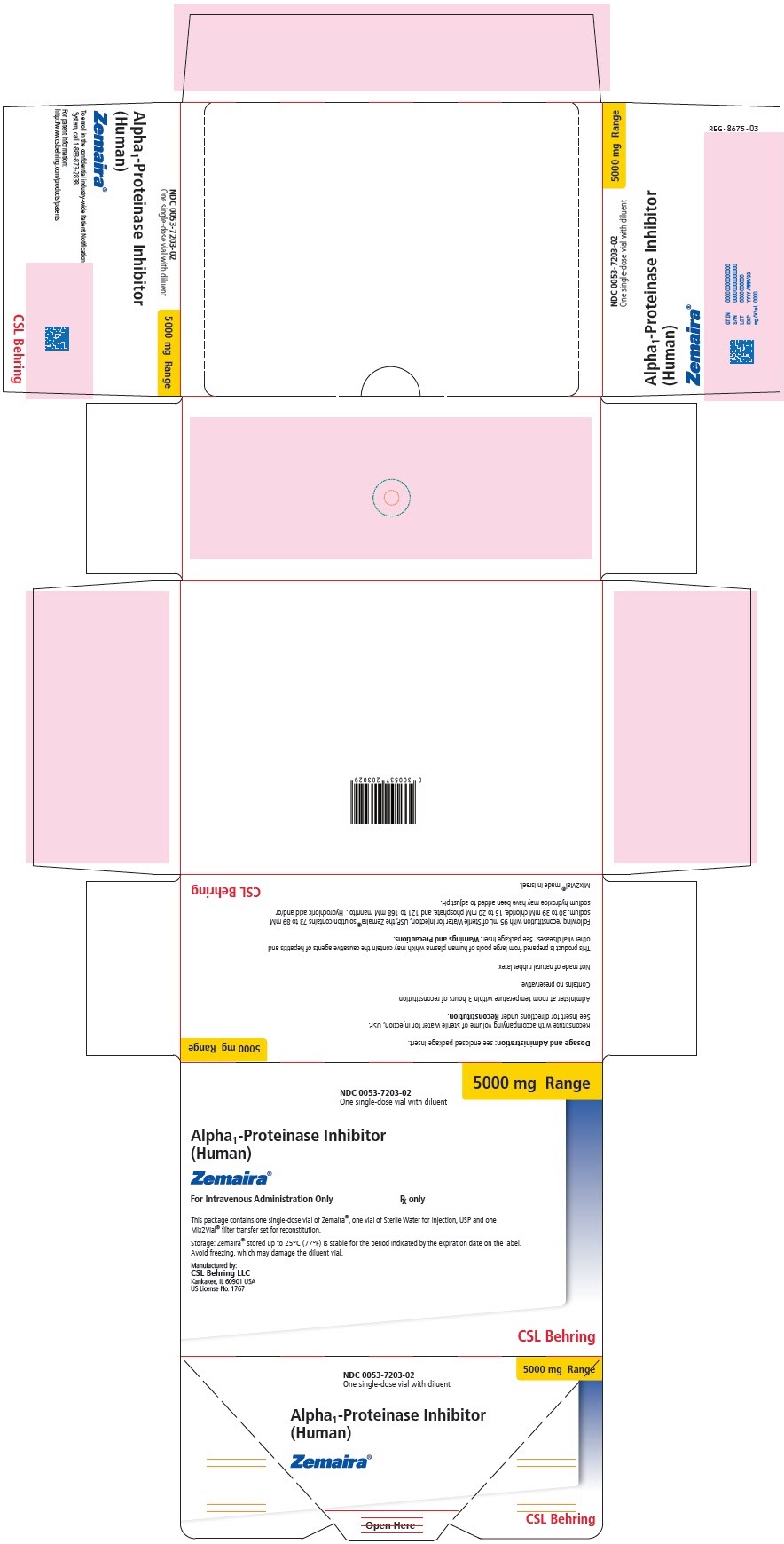
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZEMAIRA
alpha-1-proteinase inhibitor human kit kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 57516-113 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57516-113-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 95 mL Part 2 1 VIAL, SINGLE-DOSE 95 mL Part 1 of 2 ALPHA-1-PROTEINASE INHIBITOR HUMAN
alpha-1-proteinase inhibitor human injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 57516-114 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .ALPHA.1-PROTEINASE INHIBITOR HUMAN (UNII: F43I396OIS) (.ALPHA.1-PROTEINASE INHIBITOR HUMAN - UNII:F43I396OIS) .ALPHA.1-PROTEINASE INHIBITOR HUMAN 5000 mg in 95 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57516-114-01 95 mL in 1 VIAL, SINGLE-DOSE; Type 6: Drug/Biologic Combination Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125078 07/31/2023 Part 2 of 2 DILUENT
water injection injectionProduct Information Item Code (Source) NDC: 57516-115 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57516-115-20 95 mL in 1 VIAL, SINGLE-DOSE; Type 6: Drug/Biologic Combination Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125078 04/24/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125078 07/31/2023 ZEMAIRA
alpha-1-proteinase inhibitor human kit kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 57516-110 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57516-110-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 76 mL Part 2 1 VIAL, SINGLE-DOSE 76 mL Part 1 of 2 ALPHA-1-PROTEINASE INHIBITOR HUMAN
alpha-1-proteinase inhibitor human injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 57516-111 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .ALPHA.1-PROTEINASE INHIBITOR HUMAN (UNII: F43I396OIS) (.ALPHA.1-PROTEINASE INHIBITOR HUMAN - UNII:F43I396OIS) .ALPHA.1-PROTEINASE INHIBITOR HUMAN 4000 mg in 76 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57516-111-01 76 mL in 1 VIAL, SINGLE-DOSE; Type 6: Drug/Biologic Combination Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125078 07/31/2023 Part 2 of 2 DILUENT
water injection injectionProduct Information Item Code (Source) NDC: 57516-112 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57516-112-20 76 mL in 1 VIAL, SINGLE-DOSE; Type 6: Drug/Biologic Combination Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125078 07/31/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125078 07/31/2023 ZEMAIRA
alpha-1-proteinase inhibitor human kit kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 57516-101 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57516-101-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 20 mL Part 2 1 VIAL, SINGLE-DOSE 20 mL Part 1 of 2 ALPHA-1-PROTEINASE INHIBITOR HUMAN
alpha-1-proteinase inhibitor human injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 57516-102 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .ALPHA.1-PROTEINASE INHIBITOR HUMAN (UNII: F43I396OIS) (.ALPHA.1-PROTEINASE INHIBITOR HUMAN - UNII:F43I396OIS) .ALPHA.1-PROTEINASE INHIBITOR HUMAN 1000 mg in 20 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 525 mg in 20 mL SODIUM PHOSPHATE (UNII: SE337SVY37) 47 mg in 20 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM CHLORIDE (UNII: 451W47IQ8X) 44 mg in 20 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57516-102-01 20 mL in 1 VIAL, SINGLE-DOSE; Type 6: Drug/Biologic Combination Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125078 02/22/2022 Part 2 of 2 DILUENT
water injection injectionProduct Information Item Code (Source) NDC: 57516-103 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57516-103-20 20 mL in 1 VIAL, SINGLE-DOSE; Type 6: Drug/Biologic Combination Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125078 02/22/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125078 02/22/2022 Labeler - Fisher Clinical Services Inc. (199879800) Registrant - Fisher Clinical Services (079957165) Establishment Name Address ID/FEI Business Operations Fisher Clinical Services Inc. 199879800 manufacture(57516-101, 57516-110, 57516-113) , pack(57516-101, 57516-110, 57516-113) , label(57516-110, 57516-101, 57516-113)
Trademark Results [ZEMAIRA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZEMAIRA 78087711 2841496 Live/Registered |
CSL BEHRING L.L.C. 2001-10-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.