Laxative pills by Freds Inc / P & L Development, LLC DRUG FACTS
Laxative pills by
Drug Labeling and Warnings
Laxative pills by is a Otc medication manufactured, distributed, or labeled by Freds Inc, P & L Development, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
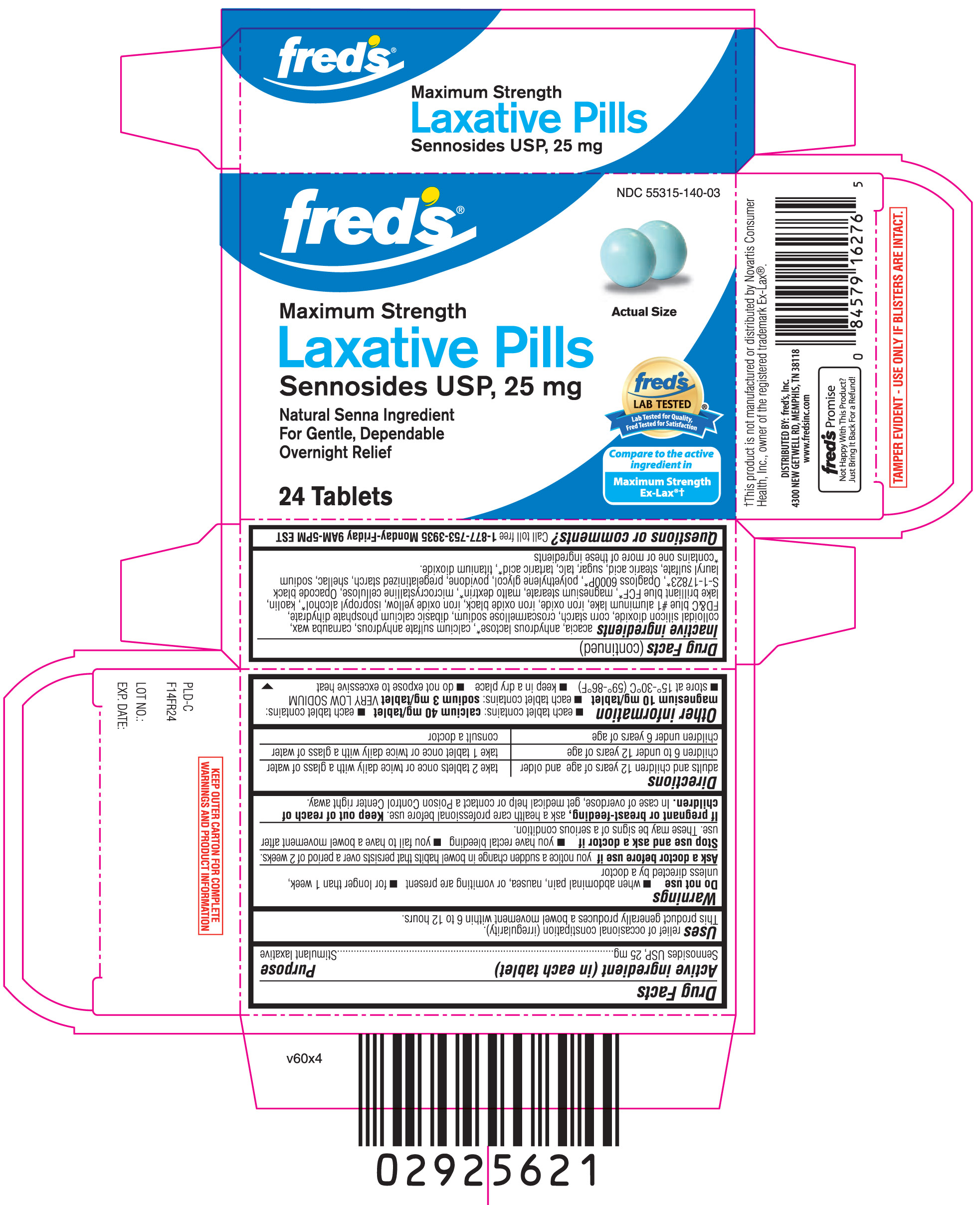
LAXATIVE PILLS MAXIMUM STRENGTH- sennosides tablet
Freds Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DRUG FACTS
Uses
- relief of occasional constipation (irregularity). This product generally produces bowel movement within 6 to 12 hours.
Warnings
Do not use
- when abdominal pain, nausea, or vomiting are present
- for longer than 1 week, unless directed by a doctor
Ask a doctor before use if
- you notice a sudden change in bowel habits that persists over a period of 2 weeks
Directions
| adults and children 12 years of age and older | take 2 tablets once or twice daily with a glass of water |
| children 6 to under 12 years of age | take 1 tablet once or twice daily with a glass of water |
| children under 6 years of age | consult a doctor |
Other information
- each tablet contains: calcium 40 mg/ tablet
- each tablet contains: magnesium 10 mg/ tablet
- each tablet contains: sodium 3 mg/ tablet VERY LOW SODIUM
- store at 15º - 30º C (59º-86º F)
- keep in a dry place
- do not expose to excessive heat
Inactive ingredients
acacia, anhydrous lactose*, calcium sulfate anhydrous, carnauba wax, colloidal silicon dioxide, corn starch, croscarmellose sodium, dibasic calcium phosphate dihydrate, FD&C Blue #1 Aluminum Lake, iron oxide, iron oxide Black, iron oxide Yellow, isopropyl alcohol*, kaolin, Lake Brilliant Blue FCF*, magnesium stearate, malto dextrin*, microcrystalline cellulose, Opacode Black S-1-17823*, Opagloss 6000P*, polyethylene glycol, povidone, pregelatinized starch, shellac, sodium lauryl sulfate, stearic acid, sugar, talc, tartaric acid*, titanium dioxide.
*contains one or more of these ingredients
Principal Display Panel
Compare to active ingredient in maximum strength EX-LAX® †
Maximum Strength
Laxative Pills
Sennosides USP, 25 mg
natural senna ingredient
for gentle, dependable overnight relief
TAMPER EVIDENT- USE ONLY IF BLISTERS ARE INTACT
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
† This product is not manufactured or distributed by Novartis Consumer Health, Inc., owner of the registered trademark Ex-Lax®
| LAXATIVE PILLS
MAXIMUM STRENGTH
sennosides tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Freds Inc (005866116) |
| Registrant - P & L Development, LLC (800014821) |