GIALAX- polyethylene glycol 3350 kit
GIALAX by
Drug Labeling and Warnings
GIALAX by is a Prescription medication manufactured, distributed, or labeled by Phlight Pharma, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- PATIENT PACKAGE INSERT
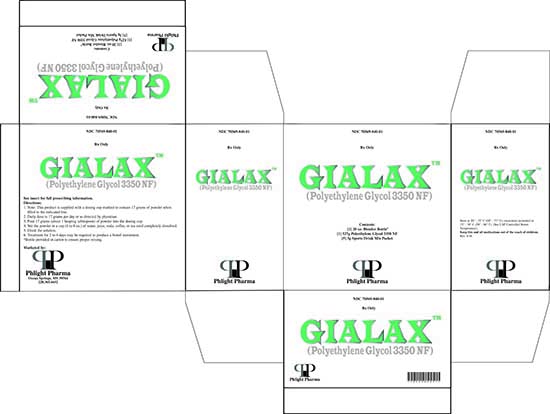
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GIALAX
polyethylene glycol 3350 kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70569-840 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70569-840-01 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 05/19/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 527 g Part 2 1 PACKET 3 g in 3 Part 1 of 2 POLYETHYLENE GLYCOL 3350
polyethylene glycol 3350 powder, for solutionProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 1 g in 1 g Product Characteristics Color white (White to Off-White) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 527 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076652 10/01/2011 Part 2 of 2 ALL SPORT MIX
sport drink mix powder, for solutionProduct Information Route of Administration ORAL Inactive Ingredients Ingredient Name Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) XANTHAN GUM (UNII: TTV12P4NEE) PIGMENT BLUE 1 (UNII: 4SBE571RQF) ASPARTAME (UNII: Z0H242BBR1) SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM CITRATE (UNII: EE90ONI6FF) MALTODEXTRIN (UNII: 7CVR7L4A2D) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) CALCIUM SILICATE (UNII: S4255P4G5M) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) MONOPOTASSIUM PHOSPHITE (UNII: 59RSS63D8J) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color Score Shape Size Flavor BLUEBERRY ("Blue Raz") Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/01/2011 Labeler - Phlight Pharma, LLC (080168751) Registrant - Phlight Pharma, LLC (080168751) Establishment Name Address ID/FEI Business Operations Phlight Pharma, LLC 080168751 label(70569-840)
Trademark Results [GIALAX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GIALAX 88245251 not registered Live/Pending |
Innovate Research & Development, LLC 2018-12-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.