Eyeone Cleaner by IDO PHARM
Eyeone Cleaner by
Drug Labeling and Warnings
Eyeone Cleaner by is a Otc medication manufactured, distributed, or labeled by IDO PHARM. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
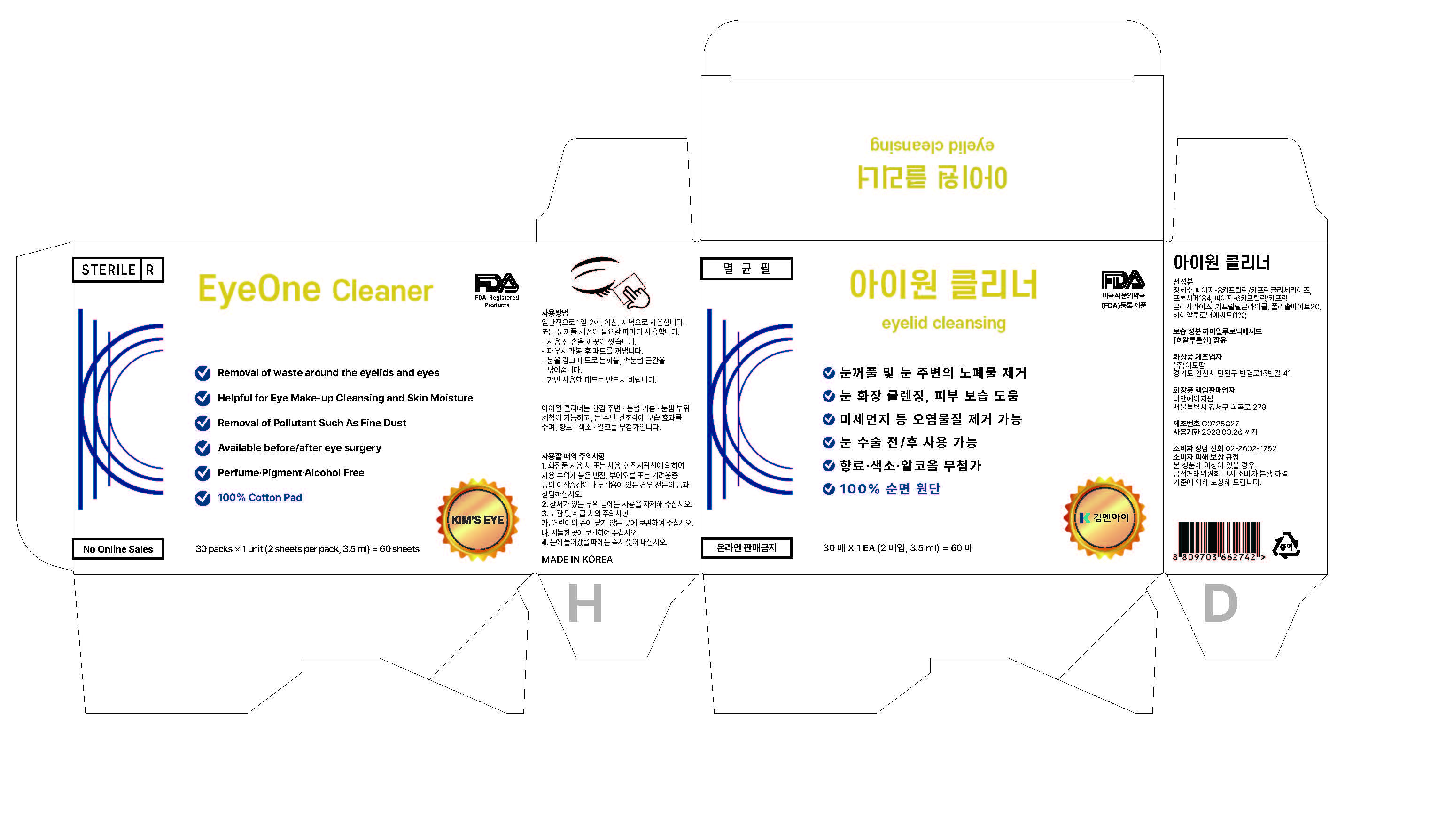
EYEONE CLEANER- hyaluronic acid cloth
IDO PHARM
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
1. Consult a specialist if you have abnormal symptoms or side effects such as red spots, swelling, or itching due to direct sunlight during or after using this product.
2. Please refrain from using the wound area
3. Precautions for Storage and Handling
A. Please keep it out of the reach of children.
B. Please store it in a cool place.
4. Wash it immediately when it gets into your eyes.
WATER, PEG-8 CAPRYLIC/CAPRIC GLYCERIDES, PEG-6 CAPRYLIC/CAPRIC GLYCERIDES, POLOXAMER 184, CAPRYLOYL GLYCINE, POLYSORBATE 20
It is generally used twice a day, in the morning and evening.
Or use it whenever you need to clean your eyelids.
- Wash your hands thoroughly before use.
- Remove the pad after opening the packet.
- Close your eyes and wipe the eyelids and the base of your eyelashes with cloth.
- Once used, throw away cloth without fail.
| EYEONE CLEANER
hyaluronic acid cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - IDO PHARM (694853523) |
| Registrant - IDO PHARM (694853523) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| IDO PHARM | 694853523 | manufacture(77039-033) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.