CENTANY AT- mupirocin ointment
Centany by
Drug Labeling and Warnings
Centany by is a Prescription medication manufactured, distributed, or labeled by Medimetriks Pharmaceuticals, Inc., Perrigo Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CENTANY® Ointment safely and effectively. See full prescribing information for CENTANY® Ointment.
CENTANY® (mupirocin) ointment, for topical use
Initial U.S. Approval: 2002INDICATIONS AND USAGE
- CENTANY® ointment is an antibacterial drug indicated for the topical treatment of impetigo due to: Staphylococcus aureus and Streptococcus pyogenes. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Ointment: 2% (3)
CONTRAINDICATIONS
- CENTANY® ointment is contraindicated in patients with a history of sensitivity reactions to any of its components. (4)
WARNINGS AND PRECAUTIONS
- Severe Allergic Reactions: Including anaphylaxis, urticaria, angioedema, and generalized rash have been reported in patients treated with CENTANY® ointment. (5.1)
- Eye and Nose Irritation: Not intended for ophthalmic use or nasal use or on mucosal surfaces. (5.2)
- Local Irritation: If signs of sensitivity or irritation should occur, treatment should be discontinued. (5.3)
- Clostridium difficile-associated Diarrhea (CDAD): If diarrhea occurs, evaluate patients for CDAD. (5.4)
- Potential for Microbial Overgrowth: Prolonged use may result in overgrowth of nonsusceptible microorganisms, including fungi. (5.5)
ADVERSE REACTIONS
Most common adverse reactions (≥0.3%) are application site reactions, pruritus, contact dermatitis, furunculosis, exfoliative dermatitis and rash. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Perrigo at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- The effect of concurrent application of CENTANY® ointment and other drug products is unknown. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe Allergic Reactions
5.2 Eye and Nose Irritation
5.3 Local Irritation
5.4 Clostridium difficile-associated Diarrhea
5.5 Potential for Microbial Overgrowth
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Severe Allergic Reactions
Systemic allergic reactions, including anaphylaxis, urticaria, angioedema, and generalized rash have been reported in patients with mupirocin formulations. [see Adverse Reactions (6.2)].
5.2 Eye and Nose Irritation
CENTANY® ointment is not for ophthalmic use or nasal use or on mucosal surfaces. If this product comes in contact with the eyes, rinse thoroughly with water.
5.3 Local Irritation
If a reaction suggesting sensitivity or chemical irritation should occur with the use of CENTANY® ointment treatment should be discontinued and appropriate alternative therapy for the infection instituted.
5.4 Clostridium difficile-associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.5 Potential for Microbial Overgrowth
As with other antibacterial products, prolonged use of CENTANY® ointment may result in overgrowth of nonsusceptible microorganisms, including fungi [see Dosage and Administration (2)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Systemic Allergic Reactions [see Warnings and Precautions (5.1)]
- Eye and Nose Irritation [see Warnings and Precautions (5.2)]
- Local Irritation [see Warnings and Precautions (5.3)]
- Clostridium difficile-associated Diarrhea [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following local adverse reactions have been reported in connection with the use of CENTANY® ointment, in 300 patients in one clinical trial: application site reactions and pruritus, each in 1% of patients; contact dermatitis and furunculosis, each in 0.7% of patients; and exfoliative dermatitis and rash, each in 0.3% of patients.
6.2 Post Marketing Experience
The following local adverse reactions have been identified during post-approval use of CENTANY® ointment. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders
Systemic allergic reactions, including anaphylaxis, urticaria, angioedema, and generalized rash [see Warnings and Precautions (5.1)].
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
There are no adequate and well-controlled studies of CENTANY® ointment in pregnant women. Because animal studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Developmental toxicity studies have been performed with mupirocin administered subcutaneously to rats and rabbits at doses up to 22 and 43 times, respectively, the human topical dose (approximately 60 mg mupirocin per day) based on body surface area. There was no evidence of fetal harm due to mupirocin.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when CENTANY® ointment is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of CENTANY® ointment have been established in the age range of 2 months to 16 years. Use of CENTANY® ointment in these age groups is supported by evidence from adequate and well-controlled studies of CENTANY® ointment in impetigo in pediatric patients studied as a part of the pivotal clinical trials [see Clinical Studies (14)].
-
11 DESCRIPTION
Each gram of CENTANY® ointment contains 20 mg mupirocin in a soft white ointment base consisting of caprylic/capric/myristic/stearic triglyceride, castor oil, oleyl alcohol, and propylene glycol monostearate. Mupirocin is a naturally occurring antibacterial drug. The chemical name is (E)-(2S,3R,4R,5S)-5-[(2S,3S,4S,5S)-2,3-Epoxy-5-hydroxy-4-methylhexyl] tetrahydro-3,4-dihydroxy-β-methyl-2H-pyran-2-crotonic acid, ester with 9-hydroxynonanoic acid. The molecular formula of mupirocin is C26H44O9 and the molecular weight is 500.62. The chemical structure is:
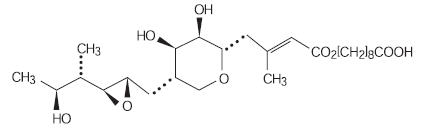
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Following the application of CENTANY® ointment to a 400cm2 area on the back of 23 healthy volunteers once daily for 7 days, the mean (range) cumulative urinary excretion of monic acid over 24 hrs following the last administration was 1.25% (0.2% to 3.0%) of the administered dose of mupirocin. The monic acid concentration in urine collected at specified intervals for 24 hrs on Day 7 ranged from <0.050 to 0.637 μg/mL.
Following intravenous or oral administration, mupirocin is rapidly metabolized. The principal metabolite, monic acid, is eliminated by renal excretion, and demonstrates no antibacterial activity. In a trial conducted in 7 healthy adult male subjects, the elimination half-life after intravenous administration of mupirocin was 20 to 40 minutes for mupirocin and 30 to 80 minutes for monic acid. The pharmacokinetics of mupirocin has not been studied in individuals with renal insufficiency.
12.4 Microbiology
Mupirocin is an antibacterial agent produced by fermentation using the organism Pseudomonas fluorescens. Mupirocin is active against some Gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA) and against some Gram-negative bacteria. Mupirocin is bactericidal at concentrations typically achieved by topical administration. The minimum bactericidal concentration (MBC) against relevant pathogens is generally eight-fold to thirty-fold higher than the minimum inhibitory concentration (MIC). In addition, mupirocin is highly protein bound (>97%), and the effect of wound secretions on the MICs of mupirocin has not been determined.
Mechanism of Action
Mupirocin inhibits bacterial protein synthesis by reversibly and specifically binding to bacterial isoleucyl transfer-RNA synthase. Due to this mode of action, mupirocin does not demonstrate cross-resistance with other classes of antimicrobial agents.
Mechanism of Resistance
Low-level mupirocin resistance (MIC = 8 - 256 mcg/mL) results from the production of a modified isoleucyl-tRNA synthase. High level resistance (MIC > 512 mcg/mL) results from acquisition, by genetic transfer, of a separate mediate isoleucyl-tRNA synthase.
Antibacterial Activity
Mupirocin has been shown to be active against susceptible strains of Staphylococcus aureus and Streptococcus pyogenes, both in vitro and in clinical studies [see Indications and Usage (1)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic potential of mupirocin have not been conducted.
Results of the following studies performed with mupirocin calcium or mupirocin sodium in vitro and in vivo did not indicate a potential for genotoxicity: rat primary hepatocyte unscheduled DNA synthesis, sediment analysis for DNA strand breaks, Salmonella reversion test (Ames), Escherichia coli mutation assay, metaphase analysis of human lymphocytes, mouse lymphoma assay, and bone marrow micronuclei assay in mice.
Reproduction studies were performed with mupirocin administered subcutaneously to male and female rats at doses up to 14 times the human topical dose (approximately 60 mg mupirocin/day) based on body surface area. Neither evidence of impaired fertility nor impaired reproductive performance attributable to mupirocin was observed.
-
14 CLINICAL STUDIES
The efficacy of topical CENTANY® ointment in impetigo was tested in one study. Patients with impetigo were randomized to receive either CENTANY® ointment or Bactroban® ointment three times daily for 7 days. Clinical efficacy rates at the follow-up visit (one week after end of therapy) in the evaluable populations (adults and pediatric patients included) were 94% for CENTANY® ointment (n=233) and 95% for Bactroban® ointment (n=242). Pathogen eradication rates at follow-up for both medications were 98%.
There were 413 pediatric patients aged 2 months to 15 years in the clinical study described above. Clinical efficacy rates at follow-up in the evaluable populations were 93% for CENTANY® ointment (n=199) and 95% for Bactroban® ointment (n=214).
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
- Use this medication only as directed by your healthcare provider.
- It is for external use only.
- Avoid contact with the eyes. If CENTANY® ointment gets in or near the eyes, rinse thoroughly with water.
- The medication should be stopped and your healthcare practitioner contacted if irritation, severe itching or rash occurs.
- If impetigo has not improved in 3 to 5 days, contact your healthcare practitioner.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 30 g Tube Kit Carton
NDC: 43538-310-30
CENTANY®
(mupirocin ointment, 2%)
ATFor Dermatologic Use Only
Rx OnlyAll-for-one Treatment
CONTENTS:
1 - 30 g Tube Centany® (mupirocin ointment, 2%)
12 - Sterile Non-Stick Gauze Pads
24 - Easy-to-Use Cloth Tape Strips (latex-free)MEDIMETRIKS
PHARMACEUTICALS, INC.
-
INGREDIENTS AND APPEARANCE
CENTANY AT
mupirocin ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 43538-310 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Mupirocin (UNII: D0GX863OA5) (mupirocin - UNII:D0GX863OA5) Mupirocin 20 mg in 1 g Inactive Ingredients Ingredient Name Strength castor oil (UNII: D5340Y2I9G) oleyl alcohol (UNII: 172F2WN8DV) propylene glycol monopalmitostearate (UNII: F76354LMGR) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43538-310-30 1 in 1 CARTON 11/15/2010 1 1 in 1 KIT 1 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050788 11/15/2010 Labeler - Medimetriks Pharmaceuticals, Inc. (019903816) Establishment Name Address ID/FEI Business Operations Perrigo Inc. 078846912 MANUFACTURE(43538-310)
Trademark Results [Centany]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CENTANY 78263336 3130516 Live/Registered |
PERRIGO ISRAEL PHARMACEUTICALS, LTD. 2003-06-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.