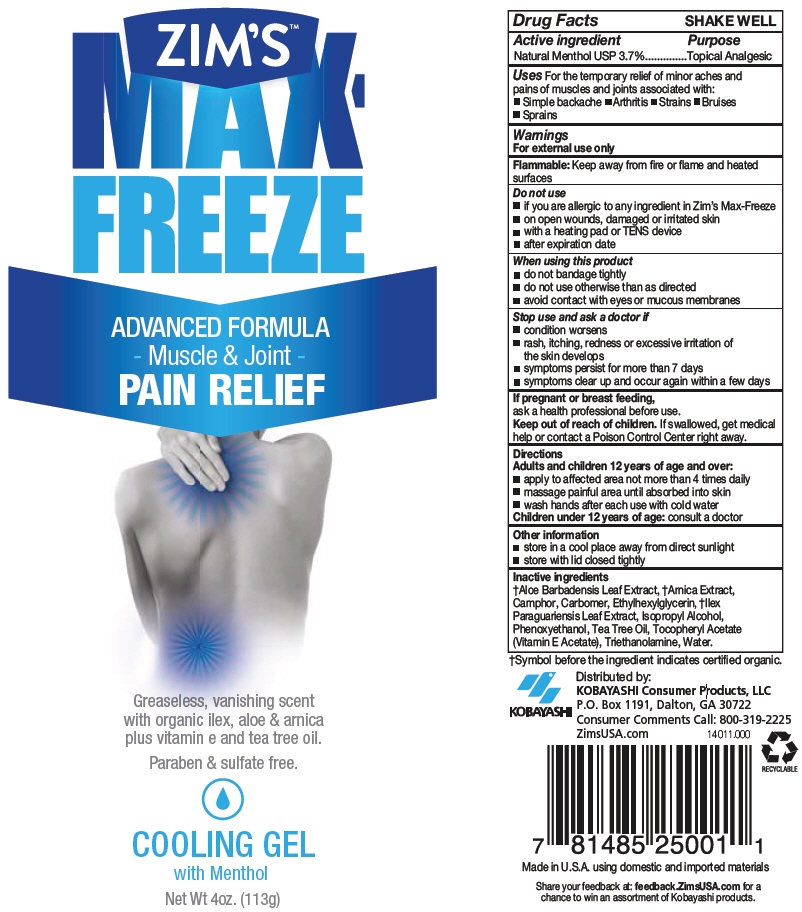
ZIMS MAX FREEZE- menthol, unspecified form gel
ZIMS MAX FREEZE by
Drug Labeling and Warnings
ZIMS MAX FREEZE by is a Otc medication manufactured, distributed, or labeled by KOBAYASHI Healthcare International, Inc., KOBAYASHI America Manufacturing, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- if you are allergic to any ingredient in Zim's Max-Freeze
- on open wounds, damaged or irritated skin
- with a heating pad or TENS device
- after expiration date
When using this product
- do not bandage tightly
- do not use otherwise than as directed
- avoid contact with eyes or mucous membranes
- Directions
- Other information
- Inactive ingredients
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 113 g Tube Label
-
INGREDIENTS AND APPEARANCE
ZIMS MAX FREEZE
menthol, unspecified form gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 54273-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 37 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) ARNICA MONTANA (UNII: O80TY208ZW) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) ISOPROPYL ALCOHOL (UNII: ND2M416302) PHENOXYETHANOL (UNII: HIE492ZZ3T) TEA TREE OIL (UNII: VIF565UC2G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54273-002-01 113 g in 1 TUBE; Type 0: Not a Combination Product 09/01/2017 2 NDC: 54273-002-02 136 g in 1 TUBE; Type 0: Not a Combination Product 09/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part348 09/01/2017 Labeler - KOBAYASHI Healthcare International, Inc. (156391729)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.