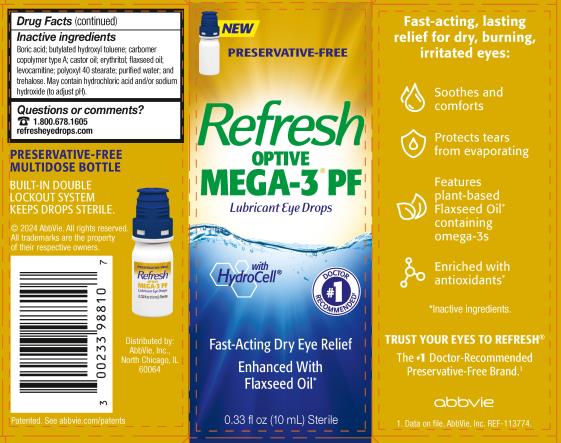
R EFRESH OPTIVE MEGA - 3 PF ® Drug Facts
Refresh Optive Mega-3 PF by
Drug Labeling and Warnings
Refresh Optive Mega-3 PF by is a Otc medication manufactured, distributed, or labeled by Allergan, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
REFRESH OPTIVE MEGA-3 PF- carboxymethylcellulose sodium, glycerin, polysorbate 80 solution/ drops
Allergan, Inc.
----------
REFRESH OPTIVE MEGA-3 PF®
Drug Facts
Uses
- For the temporary relief of burning, irritation, and discomfort due to dryness of the eye or exposure to wind or sun.
- May be used as a protectant against further irritation.
Warnings
-
For external use only.
-
To avoid contamination, do not touch tip of container to any surface. Replace cap after using.
- If solution changes color, do not use.
Directions
- Prior to first use, please read the “Instructions for Use” inside this carton.
- Instill 1 or 2 drops in the affected eye(s) as needed.
Other information
- Use only if tape seals on top and bottom flaps are intact.
- Use before expiration date marked on container.
- Discard 90 days after opening.
- Store at 59°-77°F (15°-25°C).
- RETAIN THIS CARTON FOR FUTURE REFERENCE
Inactive ingredients
Boric acid; butylated hydroxyl toluene; carbomer copolymer type A; castor oil; erythritol; flaxseed oil; levocarnitine; polyoxyl 40 stearate; purified water; and trehalose. May contain hydrochloric acid and/or sodium hydroxide (to adjust pH).
Questions or comments?
1.800.678.1605
refresheyedrops.com
PRESERVATIVE-FREE MULTIDOSE BOTTLE
BUILT-IN DOUBLE LOCKOUT SYSTEM KEEPS DROPS STERILE.
©2024 AbbVie. All rights reserved.
All trademarks are the property of their respective owners.
Distributed by: AbbVie, Inc.,
North Chicago, IL 60064
Product of US
Patented. See abbvie.com/patents
PRINCIPAL DISPLAY PANEL
NDC: 0023-3988-10
NEW
PRESERVATIVE-FREE
Refresh
OPTIVE
MEGA-3® PF
Lubricant Eye Drops
with Hydrocell®
Fast-Acting Dry Eye Relief
Enhanced With
Flaxseed Oil*
0.33 fl oz (10 mL) Sterile
| REFRESH OPTIVE MEGA-3 PF
carboxymethylcellulose sodium, glycerin, polysorbate 80 solution/ drops |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.