SHISEIDO WHITE LUCENT- ensulizole, octinoxate, and titanium dioxide cream
SHISEIDO WHITE LUCENT by
Drug Labeling and Warnings
SHISEIDO WHITE LUCENT by is a Otc medication manufactured, distributed, or labeled by SHISEIDO AMERICA INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
-
Inactive ingredients
WATER, BUTYLENE GLYCOL, GLYCERIN, DIPROPYLENE GLYCOL, HYDROGENATED POLYDECENE, PENTAERYTHRITYL TETRAETHYLHEXANOATE, DIMETHICONE, SD ALCOHOL 40-B, BEHENYL ALCOHOL, POLYBUTYLENE GLYCOL/PPG-9/1 COPOLYMER, TRANEXAMIC ACID, BEHENETH-20, TRIETHANOLAMINE, SILICA, XANTHAN GUM, PEG/PPG-14/7 DIMETHYL ETHER, ERYTHRITOL, PEG/PPG-17/4 DIMETHYL ETHER, 2-O-ETHYL ASCORBIC ACID, GLUCOSYL HESPERIDIN, PHYTOSTERYL/OCTYLDODECYL LAUROYL GLUTAMATE, SODIUM HYALURONATE, STEARYL ALCOHOL, HYDROGENATED PALM OIL, ELAEIS GUINEENSIS (PALM) KERNEL OIL, ELAEIS GUINEENSIS (PALM) OIL, SODIUM METAPHOSPHATE, TRISODIUM EDTA, CELLULOSE GUM, ALUMINUM HYDROXIDE, BHT, SODIUM METABISULFITE, TOCOPHEROL, CITRIC ACID, PHENOXYETHANOL, FRAGRANCE, IRON OXIDES
- Questions?
- SPL UNCLASSIFIED SECTION
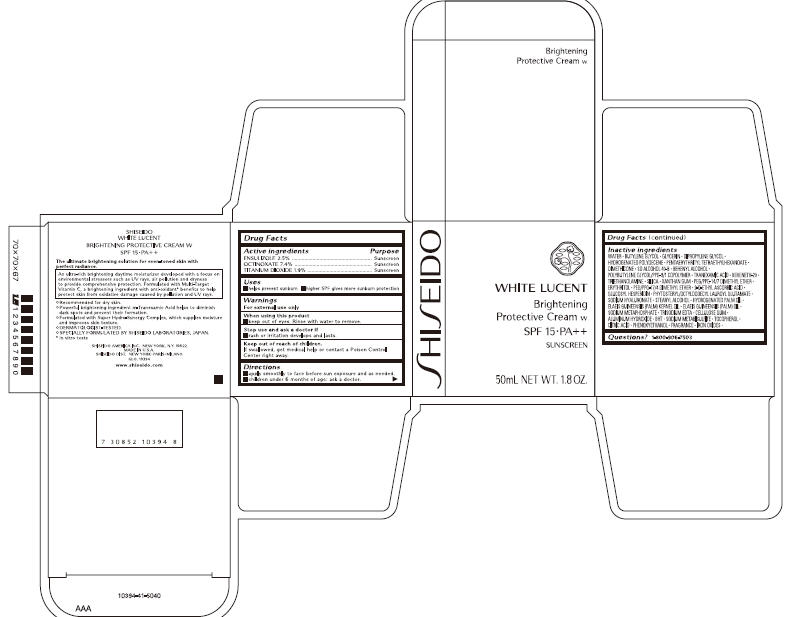
- PRINCIPAL DISPLAY PANEL - 50 mL Carton
-
INGREDIENTS AND APPEARANCE
SHISEIDO WHITE LUCENT
ensulizole, octinoxate, and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 52686-227 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENSULIZOLE (UNII: 9YQ9DI1W42) (ENSULIZOLE - UNII:9YQ9DI1W42) ENSULIZOLE 1.275 g in 51 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3.774 g in 51 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.969 g in 51 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52686-227-50 1 in 1 CARTON 02/01/2010 1 51 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/01/2010 Labeler - SHISEIDO AMERICA INC. (782677132) Establishment Name Address ID/FEI Business Operations SHISEIDO AMERICA INC. 782677132 analysis(52686-227) , manufacture(52686-227)
Trademark Results [SHISEIDO WHITE LUCENT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SHISEIDO WHITE LUCENT 76405170 3042165 Live/Registered |
Shiseido Company, Ltd. 2002-05-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.