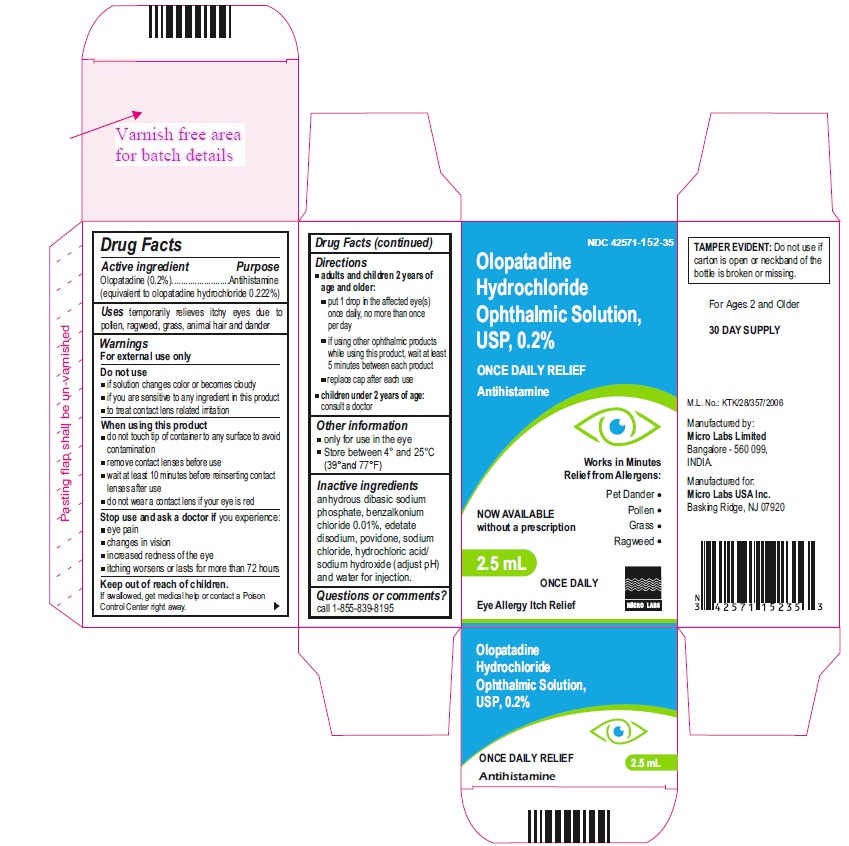
Drug Facts
OLOPATADINE HYDROCHLORIDE by
Drug Labeling and Warnings
OLOPATADINE HYDROCHLORIDE by is a Otc medication manufactured, distributed, or labeled by Micro Labs Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
OLOPATADINE HYDROCHLORIDE- olopatadine hydrochloride solution/ drops
Micro Labs Limited
----------
Drug Facts
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- do not wear a contact lens if your eye is red
Stop use and ask a doctor if you experience:
- eye pain
- changes in vision
- increased redness of the eye
- itching worsens or lasts for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
-
adults and children 2 years of age and older:
- put 1 drop in the affected eye(s) once daily, no more than once per day
- if using other ophthalmic products while using this product, wait at least 5 minutes between each product
- replace cap after each use
- children under 2 years of age: consult a doctor
Inactive ingredients
anhydrous dibasic sodium phosphate, benzalkonium chloride 0.01%, edetate disodium, povidone, sodium chloride, hydrochloric acid/sodium hydroxide (adjust pH) and water for injection.
PRINCIPAL DISPLAY PANEL
NDC: 42571-152-35
Olopatadine Hydrochloride
Opthalmic Solution
USP, 0.2%
2.5 mL
Antihistamine
Once Daily relif
Micro Labs
NDC: 42571-152-35
Olopatadine Hydrochloride
Opthalmic Solution
USP, 0.2%
2.5 mL
Antihistamine
works in minutes
Once Daily relif
Micro Labs
| OLOPATADINE HYDROCHLORIDE
olopatadine hydrochloride solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Micro Labs Limited (862174955) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.