THYRO-TABS CANINE- levothyroxine sodium tablet
Thyro-Tabs Canine by
Drug Labeling and Warnings
Thyro-Tabs Canine by is a Animal medication manufactured, distributed, or labeled by MWI/VetOne, LLOYD, Inc. of Iowa. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- WARNINGS
-
DESCRIPTION
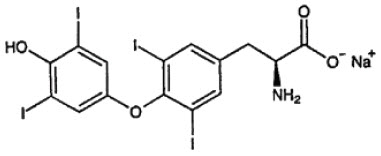
DESCRIPTION: Thyro-Tabs® Canine (levothyroxine sodium tablets), USP contains synthetic crystalline L-3,3' ,5,5'-tetraiodothyronine sodium salt [levothyroxine (T4) sodium]. Synthetic T4 is identical to that produced in the canine thyroid gland. Levothyroxine sodium has an empirical formula of C15H10I4N NaO4 ∙ H2O, molecular weight of 798.85 g/mol (anhydrous), and structural formula as shown:
- VETERINARY INDICATIONS
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION: The initial total daily dose is 0.1 mg/10 pounds (0.01 mg/lb; 0.022 mg/kg) body weight as a single dose every 24 hours or as a divided dose every 12 hours.
The dose may then be adjusted by monitoring the serum total thyroxine (TT4) concentrations 4 to 6 hours post-tablet administration, along with clinical response, of the dog every 4 to 8 weeks until an adequate maintenance dose is established.
To minimize day-to-day variations in serum TT4 concentrations (see CLINICAL PHARMACOLOGY), owners should consistently administer Thyro-Tabs Canine either with or without food.
When switching from another levothyroxine sodium formulation to Thyro-Tabs Canine, monitor serum TT4 concentrations and clinical response due to potential differences in recommended starting doses and potential differences in bioavailability.
- CONTRAINDICATIONS
-
WARNINGS:
USER SAFETY WARNINGS: Not for use in humans. Keep out of reach of children. In the event of accidental ingestion, seek medical advice immediately and show the product label to the physician. Wash hands after handling.
ANIMAL SAFETY WARNINGS: Keep Thyro-Tabs Canine in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
In humans and rodents, excess in utero exposure to thyroid hormones is associated with hypothalamic-pituitary-thyroid axis dysfunction and morphological thyroid gland defects in the offspring. The safefy of Thyro-Tabs Canine has not been evaluated In breeding, pregnant, or lactating dogs.
- PRECAUTIONS
-
ADVERSE REACTIONS:
PRE-APPROVAL EXPERIENCE: In a 6-month US field study with 92 dogs, the most commonly reported adverse reactions by percentage of dogs experiencing the reaction included: anorexia (17%), dermatitis (15%), vomiting (15%), otitis externa (14%), lethargy (14%), polydipsia (13%), diarrhea (11 %), leukocytosis (9%), pruritus (8%), tachypnea (8%), polyuria (5%), hyperactivity (4%), and seborrhea (1 %).
One dog was withdrawn from the study at the owner's request because of increased water consumption and urination, which was possibly related to levothyroxine sodium.
Hematocrit and red blood cell counts exceeded the upper limit of the reference range in seven dogs by the end of the study. Liver enzyme (ALP, ALT, or AST) elevations related to levothyroxine administration were reported in three dogs. In two of the dogs with elevated ALT and AST, the elevations resolved by Day 70 and Day 126, respectively.
POST-APPROVAL EXPERIENCE (2022): The following adverse events are based on post-approval adverse drug experience reporting forThyro-Tabs Canine. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported in dogs, are listed in decreasing order of reporting frequency: Pruritus, high or low serum thyroxine (T4) concentrations, tachypnea, weight loss, lethargy, anorexia, vomiting, polydipsia, alopecia, dermatitis, hyperactivity, diarrhea, and polyuria.
Allergic-type hypersensitivity reactions (including pruritus, hives, facial swelling, and dermatitis) have also been reported.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY: Levothyroxine sodium has poor oral bioavailability in dogs (10-20%) with peak serum TT4 concentrations wilhin 4 to 6 hours (fasted state). Administration of levothyroxine sodium with food reduces oral bioavailability.5 In most dogs, the estimated half-life is approximately 10-14 hours. Levothyroxine sodium is excreted in the feces.
-
SPL UNCLASSIFIED SECTION
EFFECTIVENESS: In a US field study with 92 dogs, dogs were administered a starling daily dose of 0.1 mg/10 lb (0.022 mg/kg) body weight. The dose was administered either once every 24 hours or as 0.05 mg/10 lb (0.011 mg/kg) body weight every 12 hours. The dose could be increased or decreased (without a change in frequency) after 6, 10, and 18 weeks, based on clinical findings and serum thyroid hormone concentrations. The maJority of the dogs (80%) were dosed at 0.08-0.12 mg/10 lb (0.008-0.012 mg/lb; 0.018-0.026 mg/kg) by the end of the study, with the majority of dogs (69.7%) requiring one dose change.
The product was considered effective if the serum TT4, free thyroxine (fT4), and thyroid stimulating hormone (TSH) concentrations were all within the desired treatment ranges when collected 4 to 6 hours post-tablet administration after 182 ± 5 days of treatment (TT4: 15-94 nmol/L; fT4: 8-36 pmol/L; TSH: ≤ 37 mU/L). Of the 78 evaluable cases, 59 (75.6%) were considered treatment successes. There was no statistical difference in lhe success rate between the once daily or divided dose treatment groups.
Clinical signs of hypothyroidism (weight gain, lethargy, bradycardia, seborrhea, alopecia, hyperpigmentation, scaling, and hypercholesterolemia) generally improved by the end of the study (Day 182 ± 5). Respiratory rate, excluding panting dogs, increased during the study along with activity level.
-
SPL UNCLASSIFIED SECTION
TARGET ANIMAL SAFETY: In a laboratory study, levothyroxine sodium was administered to 32 healthy, 7-10 month old, euthyroid Beagle dogs (4 males and 4 females per group) at 0×, 2×, 6×, and 10× the initial dose of 0.10 mg/10 lb (0.01 mg/lb) once daily for 26 weeks. Increased serum TT4 and fT4 concentrations were directly proportional to an increasing dose of levothyroxine sodium. Decreased serum TSH concentrations were inversely proportional to an increasing dose of levothyroxine sodium. Dogs treated with levothyroxine sodium had elevated red blood cell indices (hemoglobin, hematocrit, and red blood cell count) and ALT but these did not exceed the normal reference ranges. Dogs treated with levothyroxine sodium had lower albumin, calcium, globulins, and total protein values but these did not fall below the normal reference ranges. Vomiting, diarrhea, excitation, rapid respiration, tachycardia, and feces with blood were observed in all treatment groups, but were seen with greater frequency in dogs treated with levothyroxine sodium. Decreased pituitary gland and thyroid/parathyroid gland organ weights were also observed in euthyroid dogs treated with levothyroxine sodium.
- STORAGE AND HANDLING
-
HOW SUPPLIED
HOW SUPPLIED: Thyro-Tabs Canine (levothyroxine sodium tablets), USP is available as scored, color coded ovoid tablets in 9 strengths: 0.1 mg-yellow; 0.2 mg-pink; 0.3 mg-green; 0.4 mg-maroon; 0.5 mg-white; 0.6 mg-purple; 0.7 mg-orange; 0.8 mg-blue; and 1.0 mg-tan, in bottles of 120 and 1,000 tablets.
- SPL UNCLASSIFIED SECTION
-
REFERENCES
REFERENCES:
- Phillips DE, Harkin KR. Hypothyroidism and myocardial failure in two Great Danes. J Am Anim Hosp Assoc 2003;39:133-137.
- Flood JA, Hoover JP. Improvement in myocardial dysfunction in a hypothyroid dog. Can Vet J 2009;50:828-834.
- Chow B, French A. Conversion of atrial fibrillation after levothyroxine in a dog with hypothyroidism and arterial thromboembolism. J Small Anim Pract 2014;55:278-282.
- Sangster JK, Panciera DL, Abbott JA. Cardiovascular effects of thyroid disease. Compend Contin Educ Vet 2013;35:E5.
- Le Traon G, Burgaud S, Horspool LJ. Pharmacokinetics of total thyroxine in dogs after administration of an oral solution of levothyroxine sodium. J Vet Pharmacol Ther 2008;31:95-101.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 0.1 mg Tablet Bottle Label
NDC: 13985-981-20
VET one®
Thyro-Tabs® Canine
(levothyroxine sodium tablets), USP0.1 mg
For use in animals only.
Keep out of reach of children.CAUTION: Federal law restricts this drug to use by
or on the order of a licensed veterinarian.Approved by FDA under NADA # 141-448
V1 510155
1000 Tablets
-
PRINCIPAL DISPLAY PANEL - 0.2 mg Tablet Bottle Label
NDC: 13985-982-20
VET one®
Thyro-Tabs® Canine
(levothyroxine sodium tablets), USP0.2 mg
For use in animals only.
Keep out of reach of children.CAUTION: Federal law restricts this drug to use by
or on the order of a licensed veterinarian.Approved by FDA under NADA # 141-448
V1 510157
1000 Tablets
-
PRINCIPAL DISPLAY PANEL - 0.3 mg Tablet Bottle Label
NDC: 13985-983-20
VET one®
Thyro-Tabs® Canine
(levothyroxine sodium tablets), USP0.3 mg
For use in animals only.
Keep out of reach of children.CAUTION: Federal law restricts this drug to use by
or on the order of a licensed veterinarian.Approved by FDA under NADA # 141-448
V1 510159
1000 Tablets
-
PRINCIPAL DISPLAY PANEL - 0.4 mg Tablet Bottle Label
NDC: 13985-984-20
VET one®
Thyro-Tabs® Canine
(levothyroxine sodium tablets), USP0.4 mg
For use in animals only.
Keep out of reach of children.CAUTION: Federal law restricts this drug to use by
or on the order of a licensed veterinarian.Approved by FDA under NADA # 141-448
V1 510161
1000 Tablets
-
PRINCIPAL DISPLAY PANEL - 0.5 mg Tablet Bottle Label
NDC: 13985-985-20
VET one®
Thyro-Tabs® Canine
(levothyroxine sodium tablets), USP0.5 mg
For use in animals only.
Keep out of reach of children.CAUTION: Federal law restricts this drug to use by
or on the order of a licensed veterinarian.Approved by FDA under NADA # 141-448
V1 510163
1000 Tablets
-
PRINCIPAL DISPLAY PANEL - 0.6 mg Tablet Bottle Label
NDC: 13985-986-20
VET one®
Thyro-Tabs® Canine
(levothyroxine sodium tablets), USP0.6 mg
For use in animals only.
Keep out of reach of children.CAUTION: Federal law restricts this drug to use by
or on the order of a licensed veterinarian.Approved by FDA under NADA # 141-448
V1 510165
1000 Tablets
-
PRINCIPAL DISPLAY PANEL - 0.7 mg Tablet Bottle Label
NDC: 13985-987-20
VET one®
Thyro-Tabs® Canine
(levothyroxine sodium tablets), USP0.7 mg
For use in animals only.
Keep out of reach of children.CAUTION: Federal law restricts this drug to use by
or on the order of a licensed veterinarian.Approved by FDA under NADA # 141-448
V1 510167
1000 Tablets
-
PRINCIPAL DISPLAY PANEL - 0.8 mg Tablet Bottle Label
NDC: 13985-988-20
VET one®
Thyro-Tabs® Canine
(levothyroxine sodium tablets), USP0.8 mg
For use in animals only.
Keep out of reach of children.CAUTION: Federal law restricts this drug to use by
or on the order of a licensed veterinarian.Approved by FDA under NADA # 141-448
V1 510169
1000 Tablets
-
PRINCIPAL DISPLAY PANEL - 1.0 mg Tablet Bottle Label
NDC: 13985-989-20
VET one®
Thyro-Tabs® Canine
(levothyroxine sodium tablets), USP1.0 mg
For use in animals only.
Keep out of reach of children.CAUTION: Federal law restricts this drug to use by
or on the order of a licensed veterinarian.Approved by FDA under NADA # 141-448
V1 510171
1000 Tablets
-
INGREDIENTS AND APPEARANCE
THYRO-TABS CANINE
levothyroxine sodium tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13985-981 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.1 mg Product Characteristics Color YELLOW Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code 0;1;T4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13985-981-10 120 in 1 BOTTLE, PLASTIC 2 NDC: 13985-981-20 1000 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141448 11/05/2020 THYRO-TABS CANINE
levothyroxine sodium tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13985-982 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.2 mg Product Characteristics Color PINK (Light pink) Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code 0;2;T4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13985-982-10 120 in 1 BOTTLE, PLASTIC 2 NDC: 13985-982-20 1000 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141448 11/05/2020 THYRO-TABS CANINE
levothyroxine sodium tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13985-983 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.3 mg Product Characteristics Color GREEN Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code 0;3;T4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13985-983-10 120 in 1 BOTTLE, PLASTIC 2 NDC: 13985-983-20 1000 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141448 11/05/2020 THYRO-TABS CANINE
levothyroxine sodium tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13985-984 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.4 mg Product Characteristics Color PINK (Bright pink) Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code 0;4;T4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13985-984-10 120 in 1 BOTTLE, PLASTIC 2 NDC: 13985-984-20 1000 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141448 11/05/2020 THYRO-TABS CANINE
levothyroxine sodium tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13985-985 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.5 mg Product Characteristics Color WHITE Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code 0;5;T4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13985-985-10 120 in 1 BOTTLE, PLASTIC 2 NDC: 13985-985-20 1000 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141448 11/05/2020 THYRO-TABS CANINE
levothyroxine sodium tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13985-986 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.6 mg Product Characteristics Color PURPLE (Lavender) Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code 0;6;T4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13985-986-10 120 in 1 BOTTLE, PLASTIC 2 NDC: 13985-986-20 1000 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141448 11/05/2020 THYRO-TABS CANINE
levothyroxine sodium tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13985-987 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.7 mg Product Characteristics Color ORANGE (Light orange) Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code 0;7;T4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13985-987-10 120 in 1 BOTTLE, PLASTIC 2 NDC: 13985-987-20 1000 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141448 11/05/2020 THYRO-TABS CANINE
levothyroxine sodium tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13985-988 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.8 mg Product Characteristics Color BLUE (Light blue) Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code 0;8;T4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13985-988-10 120 in 1 BOTTLE, PLASTIC 2 NDC: 13985-988-20 1000 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141448 11/05/2020 THYRO-TABS CANINE
levothyroxine sodium tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13985-989 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 1 mg Product Characteristics Color BROWN (Tan) Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code 1;T4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13985-989-10 120 in 1 BOTTLE, PLASTIC 2 NDC: 13985-989-20 1000 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141448 11/05/2020 Labeler - MWI/VetOne (019926120) Registrant - LLOYD, Inc. of Iowa (007281942) Establishment Name Address ID/FEI Business Operations LLOYD, Inc. of Iowa 962286535 LABEL, PACK, MANUFACTURE Establishment Name Address ID/FEI Business Operations LLOYD, Inc. of Iowa 007281942 ANALYSIS
Trademark Results [Thyro-Tabs Canine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 THYRO-TABS CANINE 85901608 not registered Dead/Abandoned |
LLOYD, INC. OF IOWA 2013-04-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.