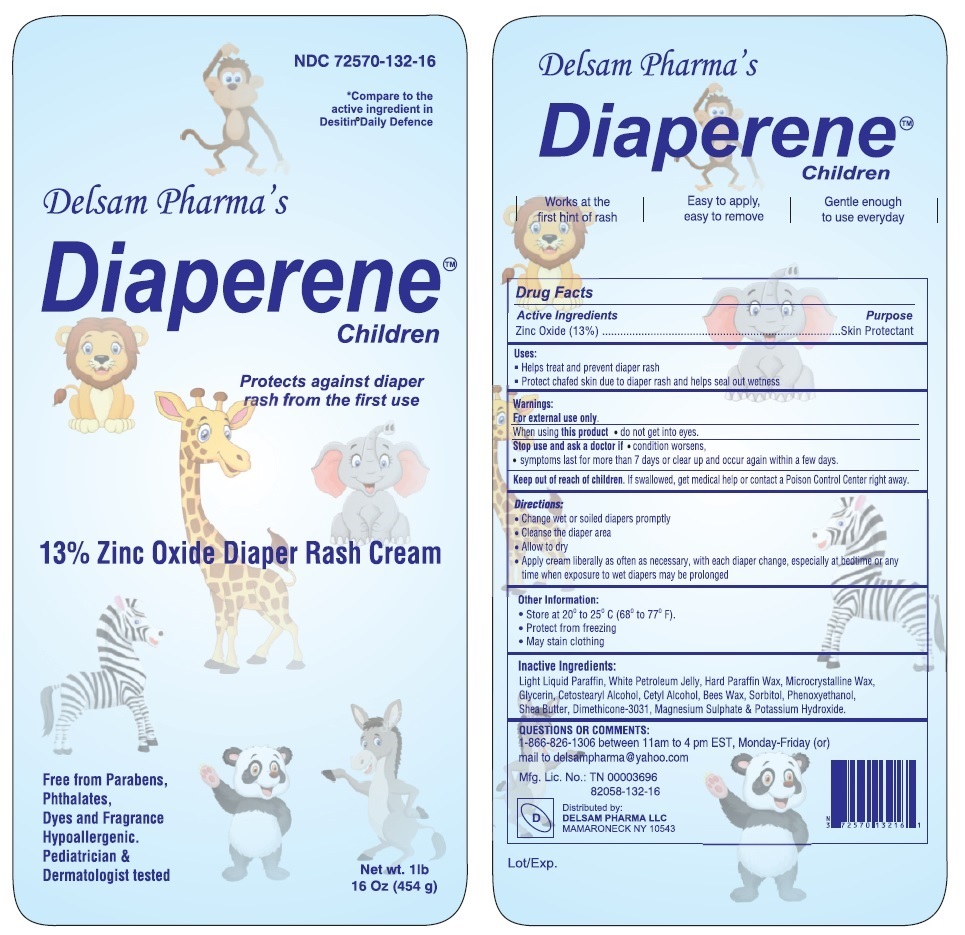
Delsam Pharma's Diaperene™ Children
Diaperene Children by
Drug Labeling and Warnings
Diaperene Children by is a Otc medication manufactured, distributed, or labeled by Delsam Pharma Llc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DIAPERENE CHILDREN- zinc oxide cream
Delsam Pharma Llc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Delsam Pharma's Diaperene™ Children
Uses:
Helps treat and prevent diaper rash
Protect chafed skin due to diaper rash and helps seal out wetness
Warnings:
For external use only.
When using this product do not get into eyes.
Stop use and ask a doctor if condition worsens,
symptoms last for more than 7 days or clear up and occur again within a few days.
Directions:
Change wet or soiled diapers promptly
Cleanse the diaper area
Allow to dry
Apply cream liberally as often as necessary, with each diaper change, especially at bedtime or any time when exposure to wet diapers may be prolonged
Inactive Ingredients:
Light Liquid Paraffin, White Petroleum Jelly, Hard Paraffin Wax, Microcrystalline Wax, Glycerin, Cetostearyl Alcohol, Cetyl Alcohol, Bees Wax, Sorbitol, Phenoxyethanol, Shea Butter, Dimethicone-3031, Magnesium Sulphate & Potassium Hydroxide.
QUESTIONS OR COMMENTS:
1-866-826-1306 between 11am to 4 pm EST, Monday-Friday (or) mail to delsampharma@yahoo.com
*Compare to the active ingredient in Desitin®Daily Defence
Protects against diaper rash from the first use
Diaper Rash Cream
Free from Parabens,
Phthalates,
Dyes and Fragrance
Hypoallergenic.
Pediatrician & Dermatologist tested
Works at the first hint of rash
Easy to apply, easy to remove
Gentle enough to use everyday
Distributed by:
DELSAM PHARMA LLC
MAMARONECK NY 10543
| DIAPERENE CHILDREN
zinc oxide cream |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Delsam Pharma Llc (081369679) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.