85248-004 complete
DERMFREE FUNGI NAIL by
Drug Labeling and Warnings
DERMFREE FUNGI NAIL by is a Otc medication manufactured, distributed, or labeled by Jiangxi Yudexi Pharmaceutical Co., LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DERMFREE FUNGI NAIL- tolnaftate 1% fungi nail liquid
Jiangxi Yudexi Pharmaceutical Co., LTD
----------
85248-004 complete
Drug Facts
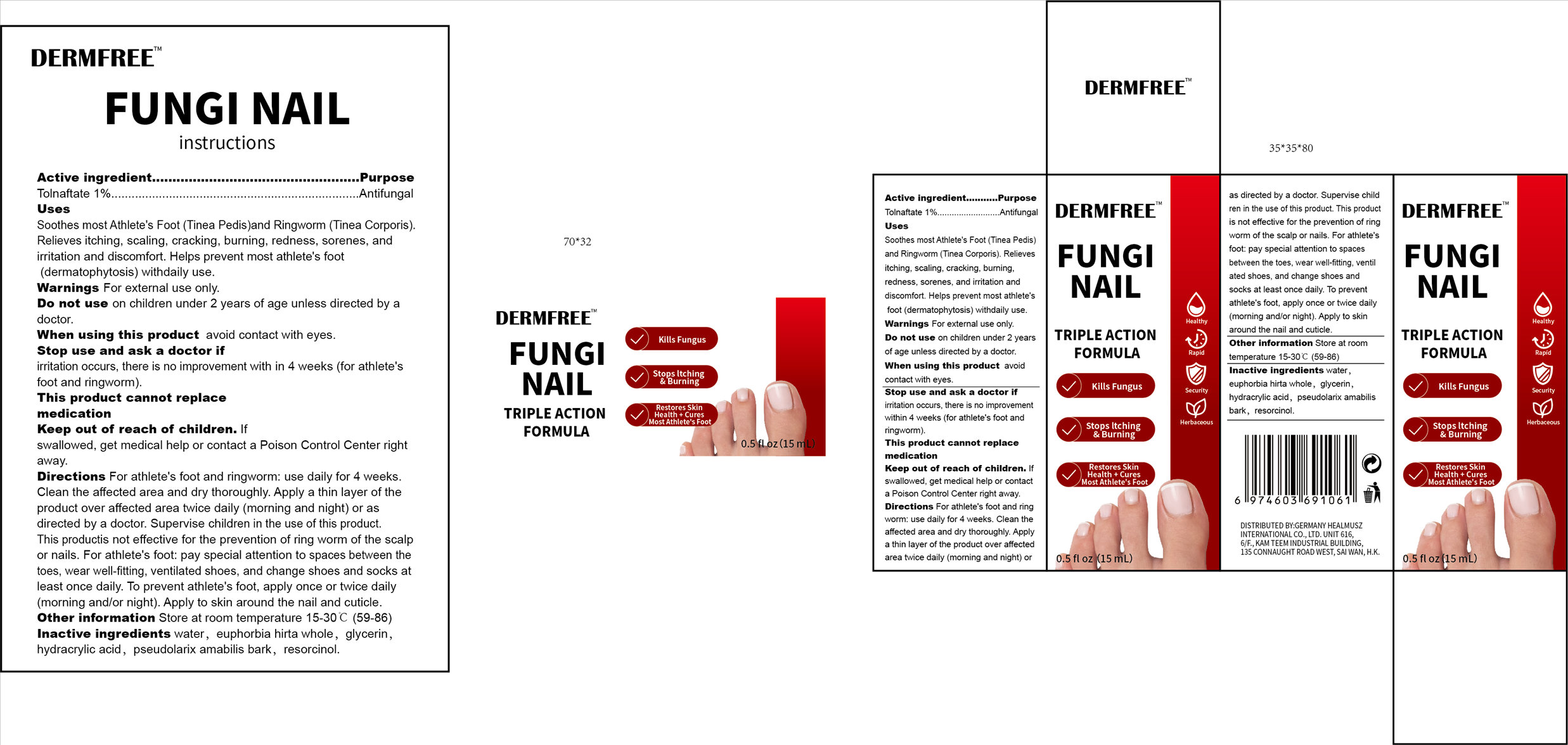
Use
Soothes most Athlete's Foot (Tinea Pedis)and Ringworm (Tinea Corporis).Relieves itching,scaling, cracking, burning, redness, sorenes, and irritation and discomfort. Helps prevent most athlete's foot(dermatophytosis) withdaily use.
Stop Use
irritation occurs, there is no improvement with in 4 weeks (for athlete's foot and ringworm).
Ask Doctor
irritation occurs, there is no improvement with in 4 weeks (for athlete's foot and ringworm).
This product cannot replace
medication
Keep Oot Of Reach Of Children
lf swallowed, get medical help or contact a Poison Control Center right away.
Directions
For athlete's foot and ringworm: use daily for 4 weeks. Clean the affected area and dry thoroughly. Apply a thin layer of the product over affected area twice daily (morning and night) or as directed by a doctor. Supervise children in the use of this product.This productis not effective for the prevention of ring worm of the scalp or nails. For athlete's foot: pay special attention to spaces between the toes, wear well-fitting, ventilated shoes, and change shoes and socks at least once daily. To prevent athlete's foot, apply once or twice daily(morning and/or night).Apply to skin around the nail and cuticle.
| DERMFREE FUNGI NAIL
tolnaftate 1% fungi nail liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Jiangxi Yudexi Pharmaceutical Co., LTD (455662836) |