SIMBADOL- buprenorphine injection
Simbadol by
Drug Labeling and Warnings
Simbadol by is a Animal medication manufactured, distributed, or labeled by Zoetis Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL Unclassified section and Caution
-
BOXED WARNING
(What is this?)
Human Safety Warning
Abuse Potential
SIMBADOL contains buprenorphine (1.8 mg/mL), an opioid agonist and Schedule III controlled substance with an abuse potential similar to other Schedule III opioids. Buprenorphine has certain opioid properties that in humans may lead to dependence of the morphine type. Abuse of buprenorphine may lead to physical dependence or psychological dependence. The risk of abuse by humans should be considered when storing, administering, and disposing of SIMBADOL. Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (suicidal depression).
Life-Threatening Respiratory Depression Respiratory depression, including fatal cases, may occur with abuse of SIMBADOL.
Additive CNS Depressant Effects
SIMBADOL has additive CNS depressant effects when used with alcohol, other opioids, or illicit drugs that cause central nervous system depression.
Accidental Exposure
Because of the potential for adverse reactions associated with accidental injection, SIMBADOL should only be administered by veterinarians or veterinary technicians who are trained in the handling of potent opioids.
See Human Safety for detailed information.
-
DESCRIPTION:
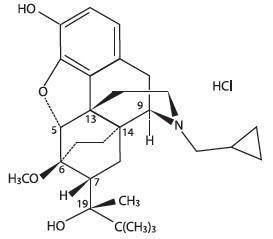
SIMBADOL is a clear, colorless to slightly yellow, sterile, injectable solution intended for subcutaneous administration for use in cats. Each milliliter of SIMBADOL contains 1.8 mg buprenorphine (equivalent to 1.94 mg buprenorphine hydrochloride), 50 mg anhydrous dextrose, 1.8 mg methylparaben, 0.2 mg propylparaben, 0.2 mg sodium acetate trihydrate, 0.5 mg glacial acetic acid, 100.0 mg anhydrous ethanol, water for injection, and hydrochloric acid and/or sodium hydroxide to adjust pH. Buprenorphine belongs to the opioid class of drugs and is a narcotic under the Controlled Substances Act due to its chemical derivation from thebaine. Buprenorphine hydrochloride is a weakly acidic, white or off-white crystalline powder with limited solubility in water. Chemically, it is 17-(cyclopropylmethyl)- α-(1,1-dimethylethyl)-4, 5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-α-methyl-6, 14-ethenomorphinan-7-methanol, hydrochloride [5α, 7α(S)]. Buprenorphine hydrochloride has the molecular formula of C29H41NO4HCl, the molecular weight of 504.09, and the following structural formula:
- INDICATION:
-
DOSAGE AND ADMINISTRATION:
The dosage of SIMBADOL is 0.24 mg/kg (0.11 mg/lb) administered subcutaneously once daily, for up to 3 days. Administer the first dose approximately 1 hour prior to surgery.
Do not dispense SIMBADOL for administration at home by the pet owner (see Human Safety ).
- CONTRAINDICATIONS:
- WARNINGS:
-
Human Safety
Not for use in humans. Keep out of reach of children.
Adult Human User Safety while handling SIMBADOL in the hospital:
Mucous membrane or eye contact during administration:
Direct contact of SIMBADOL with the eyes, oral or other mucous membranes could result in absorption of buprenorphine and the potential for adverse reactions. If accidental eye, oral or other mucous membrane contact is made during administration, flush the area with water and contact a physician.
Skin contact during administration:
If human skin is accidentally exposed to SIMBADOL, wash the exposed areas with soap and water and contact a physician. Accidental exposure could result in absorption of buprenorphine and the potential for adverse reactions.
Drug Abuse, Addiction, and Diversion of Opioids:
Controlled Substance:
SIMBADOL contains buprenorphine, a mu opioid partial agonist and Schedule III controlled substance with an abuse potential similar to other Schedule III opioids. SIMBADOL can be abused and is subject to misuse, abuse, addiction, and criminal diversion. SIMBADOL should be handled appropriately to minimize the risk of diversion, including restriction of access, the use of accounting procedures, and proper disposal methods, as appropriate to the clinical setting and as required by law.
Abuse:
Abuse of SIMBADOL poses a hazard of overdose and death. This risk is increased with concurrent abuse of alcohol and other substances including other opioids and benzodiazepines. Buprenorphine has been diverted for non-medical use into illicit channels of distribution. All people handling opioids require careful monitoring for signs of abuse. Drug abuse is the intentional non-therapeutic use of a prescription drug for its rewarding psychological or physiological effects. Abuse of opioids can occur in the absence of true addiction.
Storage and Discard:
SIMBADOL is a Class III opioid. Store in a locked, substantially constructed cabinet according to DEA and local controlled substance guidelines. Discard broached vials after 28 days. Any unused or expired vials must be destroyed by a DEA registered reverse distributor; for further information, contact your local DEA field office or call Zoetis Inc. at 1-888-963-8471.
Information for physician:
SIMBADOL injectable solution is a mu opioid partial agonist (1.8 mg buprenorphine/ mL). In the case of an emergency, provide the physician with the package insert. Naloxone may not be effective in reversing respiratory depression produced by buprenorphine. The onset of naloxone effect may be delayed by 30 minutes or more. Doxapram hydrochloride has also been used as a respiratory stimulant
-
PRECAUTIONS:
Hyperactivity (opioid excitation) has been observed up to 8 hours after anesthetic recovery (see ADVERSE REACTIONS).
Safety has not been evaluated in moribund cats (i.e., those not expected to live more than 24 hours with or without surgery). Use in such cases should be based on the risk-benefit assessment of the veterinarian.
Use with caution in cats with impaired hepatic function.
The use of SIMBADOL has not been evaluated in breeding, pregnant, or lactating cats, or in cats younger than 4 months of age.
-
ADVERSE REACTIONS:
In two controlled field studies, a total of 450 male and female cats 4 months to 16 years old, weighing between 2.6 – 20.0 lb were included in the field safety analysis. In one study, cats underwent a soft tissue surgical procedure (soft tissue). In the other study, cats underwent onychectomy, onychectomy and castration, or onychectomy and ovariohysterectomy (orthopedic). The following tables (one table for each study) show the number of cats exhibiting each observation.
Adverse Reactions in the Soft Tissue Field Study
Adverse ReactionaSimbadol (N=109) Control (N=112) During
Surgeryb
After SurgeryDuring
Surgeryb
After SurgeryHypotensionc 39 (35.8%) 29 (26.6%) 33 (29.5%) 24 (21.4%) Tachycardiad 26 (23.9%) 29 (26.6%) 15 (13.4%) 20 (17.9%) Hypothermia
(≤98.0ºF)
30 (27.5%)
1 (0.9%)
31 (27.7%)
0Hyperthermia
(≥103.0ºF)
0
40 (36.7%)
0
19 (17.0%)Hypertensione 7 (6.4%) 20 (18.3%) 9 (8.0%) 6 (5.4%) Anorexia 0 18 (16.5%) 0 15 (13.4%) Hyperactivity 0 10 (9.2%) 0 4 (3.6%) Reduced Oxygen
Saturation of
Hemoglobin (pulse
oximetry ≤90%)
5 (4.6%)
1 (0.9%)
8 (7.1%)
0Bradycardia
(≤90 beats/min)
2 (1.8%)
1 (0.9%)
1 (0.9%)
0Tachypnea
(≥72 breaths/min)
0
3 (2.8%)
0
2 (1.8%)Arrhythmia 1 (0.9%) 0 1 (0.9%) 0 Hyperesthesia 0 1 (0.9%) 0 0 Blindness 0 1 (0.9%) 0 0 Apnea/Death 0 1 (0.9%) 0 0 a. Cats may have experienced more than one type or occurrence of an adverse reaction. Cats experiencing the same reaction both during and after surgery are presented in both time periods.
b. During surgery is the time from the administration of the anesthetic induction agent until discontinuation of the gas anesthetic.
c. Hypotension is defined as a mean blood pressure of ≤60 mmHg during surgery and ≤90 mmHg after surgery.
d. Tachycardia is defined as a heart rate ≥180 beats per minute during surgery and ≥200 beats per minute after surgery.
e. Hypertension is defined as a mean blood pressure of ≥120 mmHg during surgery and ≥160 mmHg after surgery.Adverse Reactions in the Orthopedic Field Study
Adverse ReactionaSimbadol (N=115) Control (N=114) During
Surgeryb
After SurgeryDuring
Surgeryb
After SurgeryTachycardiac 29 (25.2%) 44 (38.3%) 15 (13.2%) 24 (21.1%) Hypotensiond 29 (25.2%) 22 (19.1%) 27 (23.7%) 16 (14.0%) Hyperthermia
(≥103.0ºF)
1 (0.9%)
51 (44.3%)
0
14 (12.3%)Anorexia 0 22 (19.1%) 0 20 (17.5%) Hypertensione 3 (2.6%) 20 (17.4%) 8 (7.0%) 12 (10.5%) Hypothermia
(≤98.0ºF)
8 (7.0%)
0
16 (14.0%)
0Hyperactivity 0 16 (13.9%) 0 7 (6.1%) Bradycardia
(≤90 beats/min)
3 (2.6%)
0
3 (2.6%)
1 (0.9%)Tachypnea
(≥72 beats/min)
0
2 (1.8%)
1 (0.9%)
4 (3.5%)Reduced Oxygen
Saturation of
Hemoglobin (pulse
oximetry ≤90%)
3 (2.6%)
0
3 (2.6%)
0Arrhythmia 0 1 (0.9%) 1 (0.9%) 0 Blindness 0 1 (0.9%) 0 1 (0.9%) Ataxia 0 1 (0.9%) 0 0 Apnea/Death 1 (0.9%) 0 0 0 a. Cats may have experienced more than one type or occurrence of an adverse reaction. Cats experiencing the same reaction both during and after surgery are presented in both time periods.
b. During surgery is the time from the administration of the anesthetic induction agent until discontinuation of the gas anesthetic.
c. Tachycardia is defined as a heart rate ≥180 beats per minute during surgery and ≥200 beats per minute after surgery.
d. Hypotension is defined as a mean blood pressure of ≤60 mmHg during surgery and 90 mmHg after surgery.
e. Hypertension is defined as a mean blood pressure of ≥120 mmHg during surgery and ≥160 mmHg after surgery.The two cats with apnea in the SIMBADOLTM (buprenorphine injection) group died from the adverse reaction. The cat in the soft tissue study underwent a necropsy and a specific cause of death was not found, although other remarkable findings included metastatic neoplasia affecting multiple systems. The cat in the orthopedic study experienced apnea during endotracheal intubation. The cat was healthy and a specific cause of death was not found.
Two cats in the SIMBADOL group and one cat in the placebo control group were reported with presumptive post-anesthetic cortical blindness. Both cats in the SIMBADOL group received blood pressure intervention during surgery for low blood pressure. All cats regained vision within 7 to 84 days after surgery; however, one cat in the SIMBADOL group continued to have some visual and balance deficits.
One cat in the SIMBADOL group in the soft tissue study was euthanized after completion of the study due to pulmonary complications. The complications were considered likely related to the severity of the cat’s injuries prior to surgery.
Post-Approval Experience (July, 2017):
The following adverse events are based on post-approval adverse drug experience
reporting. Not all adverse events are reported to FDA/CVM. It is not always possible
to reliably estimate the adverse event frequency or establish a causal relationship to
product exposure using these data.he following adverse events reported for cats are listed in decreasing order of
reporting frequency for SIMBADOL: Abnormal behaviors (e.g., hyperactivity,
agitation, disorientation, hiding), mydriasis, hyperthermia, anorexia, lethargy, ataxia,
and sedation.
To report suspected adverse events, for technical assistance, or to obtain a copy of
the Safety Data Sheet (SDS), contact Zoetis Inc. at 1-888-963-8471.
For additional information about adverse drug experience reporting
for animal drugs, contact the FDA at 1-888-FDA-VETS or online at
http://www.fda.gov/AnimalVeterinary/SafetyHealth. -
CLINICAL PHARMACOLOGY:
Buprenorphine is a potent, long-acting analgesic acting at opiate receptors in the central nervous system. Buprenorphine exerts its analgesic effect via high affinity binding to various subclasses of opiate receptors, particularly μ, in the central nervous system.1 Buprenorphine binds to opiate receptors with high affinity and high receptor avidity, such that its disassociation from the receptor is slow, as demonstrated in in vitro studies. This unique property of buprenorphine could account for its duration of activity.1
Following subcutaneous injection in cats, there is considerable inter-cat variability in plasma concentration and pharmacokinetic parameters.2 Formulated as an immediate release product, buprenorphine is quickly absorbed after subcutaneous injection. Pharmacological effects (e.g., mydriasis) may occur within minutes after injection. Buprenorphine plasma concentrations following subcutaneous injection did not appear to correlate to pharmacodynamics measurements (change in the thermal threshold data). In studies with SIMBADOL analgesic effects of buprenorphine appeared about one hour after injection with a 24 to 28 hour duration of action. Combined pharmacokinetic and pharmacodynamic studies have demonstrated a marked time delay between plasma concentrations and the onset and offset of the analgesic effect which is due to the slow equilibration between drug concentrations in the biophase and the slow association and dissociation of drug binding to the receptor. 2,3
Buprenorphine is metabolized in the liver. The major route of excretion of buprenorphine is in the feces. Buprenorphine undergoes oxidative metabolism by N-dealkylation to form norbuprenorphine (an active metabolite) via CYP3A4. Buprenorphine and norbuprenorphine subsequently form inactive glucuronide conjugates in the intestinal wall and the liver and its metabolites are excreted via the bile into the gastro-intestinal tract.4 The elimination half-life in cats is reported to be similar to that associated with humans and slower than that observed in dogs.5 It is also noted that because the cat is devoid of uridine diphosphate glucuronosyltransferase enzymes, conjugated metabolites may be absent.5
-
EFFECTIVENESS:
The effectiveness of SIMBADOL was demonstrated in two randomized, masked, placebo-controlled, multi-site field studies involving client-owned cats of various breeds. In one study (soft tissue), 221 cats underwent a soft tissue surgical procedure. In the other study (orthopedic), 229 cats underwent an onychectomy alone or in combination with castration or ovariohysterectomy. Cats received either a subcutaneous injection of 0.24 mg/kg of SIMBADOL or physiologic saline approximately 60 minutes prior to surgery at the same time as pre-anesthetic medication. SIMBADOL or physiologic saline was given once daily for two additional treatments 24 and 48 hours after the initial treatment. A descriptive, interactive pain assessment system was used by the trained assessor over the 72 hour post-operative period to determine pain control. Treatment success was defined as a cat that made it through the entire 72 hour period without rescue analgesia. In the soft tissue field study, a statistically significant difference in the proportion of treatment successes in the SIMBADOL treatment group (66/93 or 71.0%) compared to the placebo control group (45/102 or 44.1%) was observed. Twenty-seven out of 93 (29.0%) SIMBADOL cases and 57 out of 102 (55.9%) placebo cases were treatment failures. In the orthopedic field study, a statistically significant difference in the proportion of treatment successes in the SIMBADOL treatment group (64/105 or 61.0%) compared to the placebo control group (33/102 or 32.4%) was observed. Forty-one out of 105 (39.0%) SIMBADOL cases and 69 out of 102 (67.6%) placebo cases were treatment failures. For both studies, the majority of the treatment failures required rescue within 4 hours after anesthetic recovery.
Combining both studies (450 cats), sedation was observed in 68 cats in the buprenorphine group and 62 cats in the placebo control group for up to 4 hours after anesthetic recovery. In both studies, during surgery, mean respiratory rates and mean blood pressures were lower in the buprenorphine group compared to the placebo control group. There were a higher number of cats and a higher number of incidences of pain on injection in the buprenorphine group (20 cats, 28 incidences) compared to the placebo control group (8 cats, 10 incidences).
The results of two field studies demonstrate that SIMBADOL is effective and has an acceptable safety margin for the control of postoperative pain in cats.
-
ANIMAL SAFETY:
Nine-Day Target Animal Safety Study: In a 9 day safety study, 4 month old healthy cats (4/sex/group) were administered SIMBADOL subcutaneously at 0X (saline), 1X (0.24 mg/kg), 3X (0.72 mg/kg), or 5X (1.2 mg/kg) once daily. All 32 cats survived to study termination.
Buprenorphine-related clinical observations included difficulty in handling, lower incidence of urination, abnormal oral dryness, dilated pupils, and decreased pupillary light reflex. The incidence of temperatures ≥103°F was higher in the buprenorphine-treated groups compared to the control group. The highest temperature observed in the buprenorphine-treated group was 103.8°F in a 5X cat.
One 1X cat and one 3X cat experienced an episode of hyperactivity, difficulty in handling, slight disorientation, agitation, dilated pupils (which were responsive to light), and respiratory sinus arrhythmia. One 1X cat (one episode) and one 3X cat (three episodes) were reported with nystagmus. One 1X and one 3X cat were reported with decreased blink response (one episode). Three cats in the 5X group lost body weight (79 g or less) during the study which correlated with decreased food consumption. All other cats gained weight during the study.
The incidence of “moderate responses” (minor vocalization or wincing and quick resolution) and “severe responses” (tried to bite or scratch or had marked vocalization or persistent attention to the injection site) to injection was higher in the buprenorphine-treated groups compared to the control group.
Respiratory rate, heart rate, and blood pressure were similar between all groups, including the control group.
Buprenorphine-related clinical pathology findings included an increase in creatine kinase values in the 3X and 5X groups and correlated with subcutaneous inflammation at the injection sites.
Histologic lesions included minimal to moderate subacute inflammation at the injection sites, which correlated with the administration of buprenorphine compared to the control group. The incidence of inflammation was similar between buprenorphine-treated groups; however, more sites with mild and moderate inflammation were observed in the 5X group compared to the 1X and 3X groups where more sites with minimal inflammation were observed. Mineralization at an injection site was seen in one 1X and one 3X cat. Chronic inflammation in the heart (valve or myocardium) was seen in two 5X cats. Subacute liver inflammation was seen in one control cat, two 1X cats, three 3X cats, and three 5X cats. Lymphoid hyperplasia of the mediastinal lymph node was seen in one 1X cat, and acute inflammation was seen in the mediastinal lymph node of one 3X cat. Lymphoid hyperplasia of the Peyer’s Patches was seen in two 1X cats and one 5X cat. Lymphoid hyperplasia, lymphocytic infiltrate, or subacute inflammation of the stomach was seen in four 1X cats, four 3X cats, and three 5X cats. Subacute inflammation or lymphocytic infiltrate of the thyroid glands was seen in two 1X cats, one 3X cat, and four 5X cats.
Arterial Blood Pressure Study in Cats: Healthy 8.5 to 29.1 month old cats (4/sex/group) were subcutaneously administered SIMBADOL at 0.24 mg/kg (1X) or meloxicam (control), 1 hour prior to anesthetic induction for a 1 hour exploratory laparotomy. Arterial blood pressure was monitored following anesthetic induction and through laparotomy, with indirect blood pressure monitoring prior to anesthesia and for 8 hours following anesthetic recovery. All 16 animals were clinically healthy for the duration of the study. There were no differences between treatment groups in mean blood pressure during the study.
During surgery and postoperatively, heart rate was higher for the buprenorphine group. During surgery, the incidence of heart rates ≥180 beats/minute was higher in the buprenorphine-treated group compared to the control group. Post-operatively, the incidence of heart rates ≥200 beats/minute was higher in the buprenorphine-treated group compared to the control group. During surgery, respiration rate was lower for the buprenorphine group. Post-operatively, body temperature was higher for the buprenorphine group. Four cats in the buprenorphine group had temperatures ≥103°F post-operatively compared to none in the control group. The highest temperature observed in the buprenorphine group was 104.3°F. Electrocardiograms were qualitatively normal in all cats. During surgery, one cat in the buprenorphine group had hemoglobin saturation less than 90% (88% at one time point).
- STORAGE INFORMATION:
- HOW SUPPLIED:
-
REFERENCES:
REFERENCES:
1. Heel RC, Brogden RN, Speight TM, et al. Buprenorphine: a review of its pharmacological
properties and therapeutic efficacy. Drugs 1979;17:81-110.
2. Steagall PV, Pelligand L, Giordano T, et al. Pharmacokinetic and pharmacodynamic modelling of
intravenous, intramuscular and subcutaneous buprenorphine in conscious cats. Vet Anaesth Analg
2013;40:83-95.
3. Robertson SA, Lascelles BD, Taylor PM, et al. PK-PD modeling of buprenorphine in cats:
intravenous and oral transmucosal administration. J Vet Pharmacol Ther 2005;28:453-460.
4. Brewster D, Humphrey MJ, McLeavy MA. Biliary excretion, metabolism and enterohepatic circulation
of buprenorphine. Xenobiotica 1981;11:189-196.
5. Court MH. Feline drug metabolism and disposition: pharmacokinetic evidence for species
differences and molecular mechanisms. Vet Clin Small Anim 2013;43: 1039-1054.Distributed by: Zoetis Inc.
Kalamazoo, MI 49007 Product of United KingdomRevised: July 2017
50270801
-
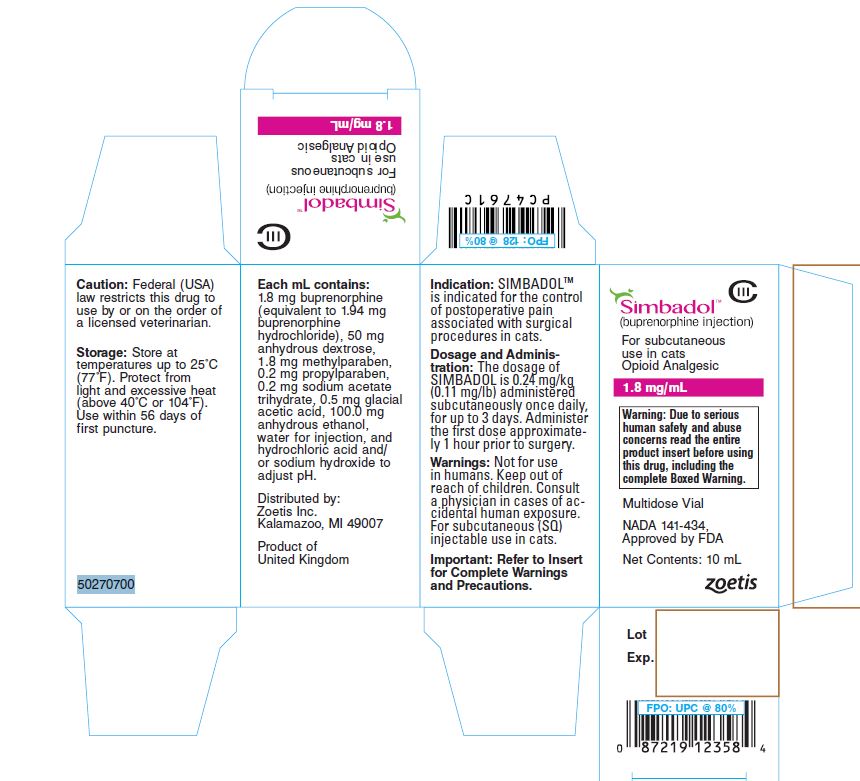
Principal Display Panel - Carton
(Simbadol Logo)
Simbadol™
(buprenorphine injection)
For subcutaneous
use in cats
Opioid Analgesic
1.8 mg/mlWarning: Due to serious human safety and
abuse concerns read the entire product
insert before using this drug, including the
complete Boxed Warning.Multidose Vial
NADA 141-434,
Approved by FDANet Contents: 10 ml
50270700
Zoetis
-
INGREDIENTS AND APPEARANCE
SIMBADOL
buprenorphine injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 54771-4131 Route of Administration SUBCUTANEOUS DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPRENORPHINE HYDROCHLORIDE (UNII: 56W8MW3EN1) (BUPRENORPHINE - UNII:40D3SCR4GZ) BUPRENORPHINE 1.8 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) 50 mg in 1 mL METHYLPARABEN (UNII: A2I8C7HI9T) 1.8 mg in 1 mL PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.2 mg in 1 mL SODIUM ACETATE (UNII: 4550K0SC9B) 0.2 mg in 1 mL ACETIC ACID (UNII: Q40Q9N063P) 0.5 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) 100.0 mg in 1 mL WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54771-4131-1 1 in 1 CARTON 1 10 mL in 1 VIAL, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141434 08/18/2015 Labeler - Zoetis Inc. (828851555)
Trademark Results [Simbadol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SIMBADOL 85949880 4679497 Live/Registered |
Abbott Laboratories 2013-06-04 |
 SIMBADOL 85655693 4625350 Live/Registered |
ZOETIS BELGIUM S.A. 2012-06-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.