ACID REDUCER- omeprazole tablet, delayed release
Acid Reducer by
Drug Labeling and Warnings
Acid Reducer by is a Otc medication manufactured, distributed, or labeled by Rugby Laboratories, Aurohealth LLC, Aurobindo Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Tips for Managing Heartburn
- Do not lie flat or bend over after eating
- Do not wear tight-fitting clothing around the stomach
- Do not eat before bedtime
- Raise the head of your bed
- Avoid heartburn-causing foods such as rich, spicy, fatty or fried foods, chocolate, caffeine, alcohol and certain fruits and vegetables
- Eat slowly and avoid big meals
- If overweight, lose weight
- Quit smoking
- Drug Facts
- Active ingredient (in each tablet)
- Purpose
- Use
- Warnings
-
Do not use if you have:
- trouble or pain swallowing food, vomiting with blood, or bloody or black stools
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
These may be signs of a serious condition. See your doctor.
- Ask a doctor before use if you have:
- ASK DOCTOR/PHARMACIST
- Stop use and ask a doctor if:
- PREGNANCY OR BREAST FEEDING
- Keep out of reach of children.
-
Directions
- for adults 18 years of age and older
- this product is to be used once a day (every 24 hours), every day for 14 days
- it may take 1 to 4 days for full effect; some people get complete relief of symptoms within 24 hours
14-Day Course of Treatment
- swallow 1 tablet with a glass of water before eating in the morning
- take every day for 14 days
- do not take more than 1 tablet a day
- do not use for more than 14 days unless directed by your doctor
- swallow whole. Do not chew or crush tablets.
Repeated 14-Day Courses (if needed)
- you may repeat a 14-day course every 4 months
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- children under 18 years of age: ask a doctor. Heartburn in children may sometimes be caused by a serious condition.
- Other information
-
Inactive ingredients
crospovidone, glyceryl monostearate, hydroxypropyl cellulose, hypromellose, magnesium stearate, methacrylic acid and ethyl acrylate copolymer dispersion, microcrystalline cellulose, polyethylene glycol, polysorbate 80, red iron oxide, silicified microcrystalline cellulose, sodium hydroxide, sodium stearyl fumarate, sugar spheres [which contains liquid glucose, starch (maize) and sucrose], talc, titanium dioxide, triethyl citrate and yellow iron oxide.
- Questions or comments?
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg (14 Tablet Bottle)
Rugby® NDC: 0536-1322-88
omeprazole
delayed-release tablets
see current Drug Facts
omeprazole delayed-release tablets 20 mg
acid reducer
24 hour
- treats frequent heartburn
14 tablets one 14-day course of treatment
may take 1 to 4 days for full effect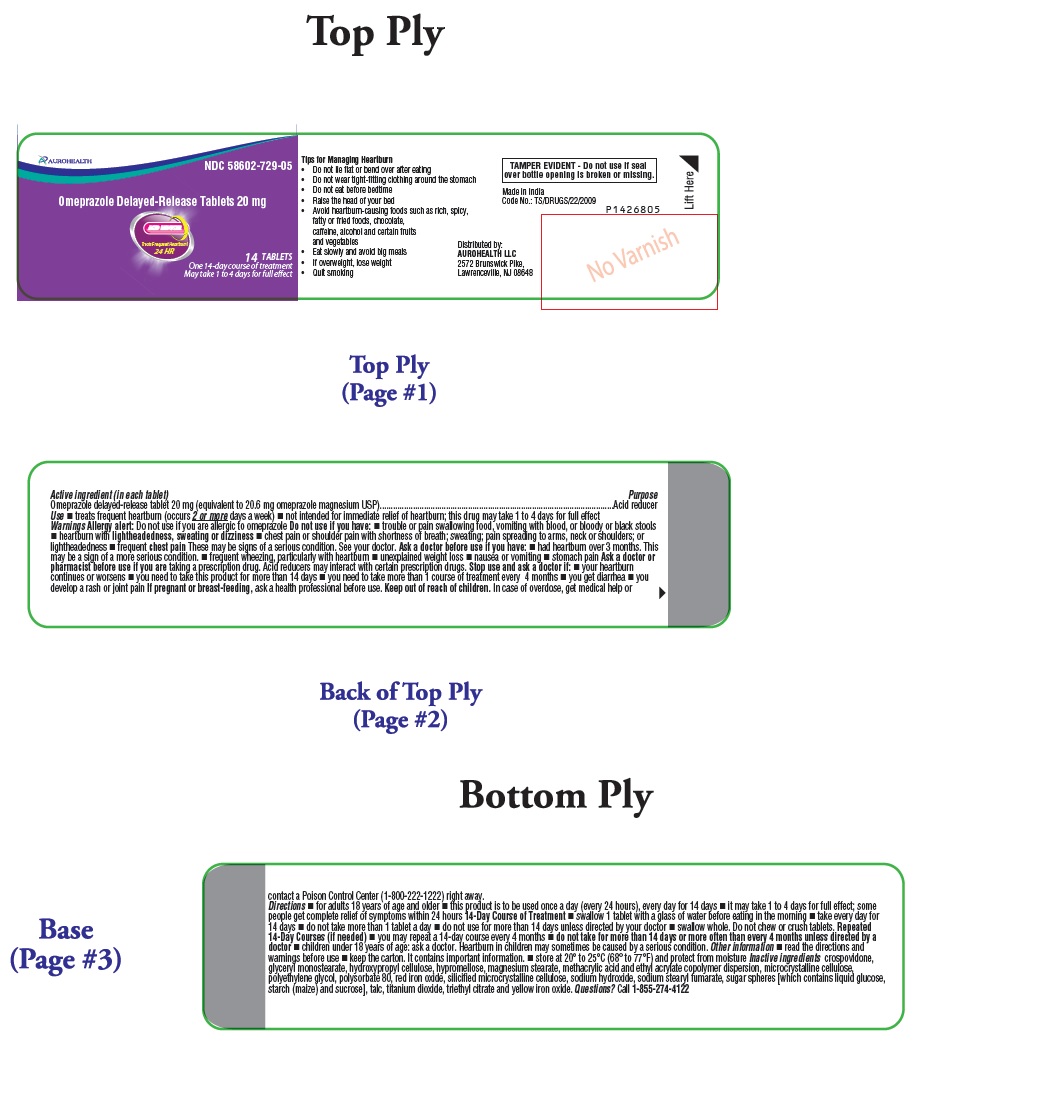
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg Container Carton Label
Rugby® NDC: 0536-1322-71
Compare to the active ingredient
in Prilsec OTC®*omeprazole
delayed-release tablets
see current Drug Facts
omeprazole delayed-release tablets 20 mg
acid reducer
24 hour
- treats frequent heartburn actual size
28 tablets two 14-day courses of treatment
may take 1 to 4 days for full effect
-
INGREDIENTS AND APPEARANCE
ACID REDUCER
omeprazole tablet, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0536-1322 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OMEPRAZOLE MAGNESIUM (UNII: 426QFE7XLK) (OMEPRAZOLE - UNII:KG60484QX9) OMEPRAZOLE 20 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (35 .MU.M) (UNII: 40UAA97IT9) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HYDROXYPROPYL CELLULOSE (90000 WAMW) (UNII: UKE75GEA7F) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) POLYSORBATE 80 (UNII: 6OZP39ZG8H) FERRIC OXIDE RED (UNII: 1K09F3G675) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYDROXYPROPYL CELLULOSE (45000 WAMW) (UNII: 8VAB711C5E) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) Product Characteristics Color PINK Score no score Shape RECTANGLE (Oblong) Size 14mm Flavor Imprint Code Z;69 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0536-1322-88 1 in 1 CARTON 09/15/2020 1 14 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 0536-1322-71 2 in 1 CARTON 09/15/2020 2 14 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 0536-1322-13 3 in 1 CARTON 09/15/2020 3 14 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206877 09/15/2020 Labeler - Rugby Laboratories (079246066) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650381903 ANALYSIS(0536-1322) , MANUFACTURE(0536-1322)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.