Temozolomide by Ascend Laboratories, LLC / Deva Holding A.S TEMOZOLOMIDE capsule
Temozolomide by
Drug Labeling and Warnings
Temozolomide by is a Prescription medication manufactured, distributed, or labeled by Ascend Laboratories, LLC, Deva Holding A.S. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TEMOZOLOMIDE CAPSULES, USP safely and effectively. See full prescribing information for TEMOZOLOMIDE CAPSULES, USP.
TEMOZOLOMIDE Capsules for oral use
Initial U.S. Approval: 1999
RECENT MAJOR CHANGES
Warnings and Precautions (5.5) 12/2019
INDICATIONS AND USAGE
TEMOZOLOMIDE Capsules, USP are an alkylating drug indicated for the treatment of adult patients with:
Newly diagnosed glioblastoma concomitantly with radiotherapy and then as maintenance treatment. (1.1)
Refractory anaplastic astrocytoma who have experienced disease progression on a drug regimen containing nitrosourea and procarbazine. (1.2)
DOSAGE AND ADMINISTRATION
- Newly Diagnosed Glioblastoma:
o 75 mg/m2 once daily for 42 days concomitant with focal radiotherapy followed by initial maintenance dose of 150 mg/m2 once daily for Days 1 to 5 of each 28-day cycle for 6 cycles. May increase maintenance dose to 200 mg/ m2 for cycles 2 – 6 based on toxicity. (2.1)
o Provide Pneumocystis pneumonia (PCP) prophylaxis during concomitant phase and continue in patients who develop lymphopenia until resolution to grade 1 or less. (2.1) - Refractory Anaplastic Astrocytoma: Initial dose of 150 mg/m2 once daily on Days 1 to 5 of each 28-day cycle. (2.2)
DOSAGE FORMS AND STRENGTHS
· 5-mg, 20-mg, 100-mg, 140-mg, 180-mg, and 250-mg capsules. (3)
CONTRAINDICATIONS
History of hypersensitivity to temozolomide or any other ingredients in TEMOZOLOMIDE capsules, USP and dacarbazine.(4.1)
WARNINGS AND PRECAUTIONS
- Myelosuppression: Monitor absolute neutrophil count (ANC) and platelet count prior to each cycle and during treatment. Geriatric patients and women have a higher risk of developing myelosuppression. (5.1)
- Myelodysplastic Syndrome and Secondary Malignancies, including myeloid leukemia, have been observed. (5.2)
- Pneumocystis Pneumonia (PCP): Closely monitor all patients, particularly those receiving steroids, for the development of lymphopenia and PCP.(5.3)
- Hepatotoxicity: Fatal and severe hepatotoxicity have been reported. Perform liver function tests at baseline, midway through the first cycle, prior to each subsequent cycle, and approximately 2 to 4 weeks after the last dose of TEMOZOLOMIDE (5.4)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. Advise male patients with pregnant partners or female partners of reproductive potential to use condoms. (5.5, 8.1, 8.3)
ADVERSE REACTIONS
- The most common adverse reactions (≥ 20% incidence) are: alopecia, fatigue, nausea, vomiting, headache, constipation, anorexia, and convulsions. (6.1)
- The most common Grade 3 to 4 hematologic laboratory abnormalities (≥ 10% incidence) in patients with anaplastic astrocytoma are: decreased lymphocytes, decreased platelets, decreased neutrophils, and decreased leukocytes.(6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ascend Laboratories LLC at 1-877-ASC-RX01 (877-272-7901) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2019
- Newly Diagnosed Glioblastoma:
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Newly Diagnosed Glioblastoma
1.2 Refractory Anaplastic Astrocytoma
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage and Dosage Modifications for Newly Diagnosed Glioblastoma
2.2 Recommended Dosage and Dosage Modifications for Refractory Anaplastic Astrocytoma
2.3 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
5.2 Myelodysplastic Syndrome and Secondary Malignancies
5.3 Pneumocystis Pneumonia
5.4 Hepatotoxicity
5.5 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Newly Diagnosed Glioblastoma
14.2 Refractory Anaplastic Astrocytoma
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage and Dosage Modifications for Newly Diagnosed Glioblastoma
Administer TEMOZOLOMIDE either orally once daily for 42 consecutive days during the concomitant phase with focal radiotherapy and then once daily on Days 1 to 5 of each 28-day cycle for 6 cycles during the maintenance phase.
Provide Pneumocystis pneumonia (PCP) prophylaxis during the concomitant phase and continue in patients who develop lymphocytopenia until resolution to grade 1 or less [see Warnings and Precautions (5.3)].
Concomitant Phase
The recommended dosage of TEMOZOLOMIDE is 75 mg/m2 either orally or intravenously once daily for 42 days (up to 49 days) concomitant with focal radiotherapy (60 Gy administered in 30 fractions). Focal radiotherapy includes the tumor bed or resection site with a 2- to 3-cm margin.
Obtain a complete blood count weekly. No dose reductions are recommended during the concomitant phase. The recommended dosage modifications during the concomitant phase are provided in Table 1.
TABLE 1: Temozolomide Dosage Modifications During Concomitant Phase
Adverse Reaction
Interruption
Discontinuation
Absolute Neutrophil Count
Withhold TEMOZOLOMIDE if ANC is greater than or equal to 0.5 x 109/L and less than 1.5 x 109/L. Resume TEMOZOLOMIDE when ANC is greater than or equal to 1.5 x 109/L.
Discontinue TEMOZOLOMIDE if platelet count is less than 0.5 x 109/L.
Platelet Count
Withhold TEMOZOLOMIDE if platelet count is greater than or equal to 10 x 109/L and less than 100 x 109/L. Resume TEMODAR when platelet count is greater than or equal to 100 x 109/L.
Discontinue TEMOZOLOMIDE if platelet count is less than 10 x 109/L.
Non-hematological Adverse Reaction (except for alopecia, nausea, vomiting)
Withhold TEMOZOLOMIDE if Grade 2 adverse reaction occurs. Resume TEMODAR when resolution to Grade 1 or less.
Discontinue TEMOZOLOMIDE if Grade 3 or 4 adverse reaction occurs.
Maintenance Phase
Beginning 4 weeks after Concomitant Phase completion, administer TEMOZOLOMIDE either orally or intravenously once daily on Days 1 to 5 of each 28-day cycle for 6 cycles. The recommended dosage of TEMOZOLOMIDE is as follows:
Cycle 1: 150 mg/m2 per day
Cycles 2 to 6: May increase to 200 mg/m2 per day if the following conditions are met before starting cycle 2. If the dose was not escalated at the onset of Cycle 2, do not increase the dose for Cycles 3 to 6.
o Nonhematologic toxicity is grade 2 or less (except for alopecia, nausea, and vomiting)
o ANC is greater than or equal to 1.5 x 109/L and
o Platelet count is greater than or equal to 100 x 109/L.
Obtain a complete blood count on Day 22 and then weekly until the ANC is above 1.5 x 109/L and the platelet count is above 100 x 109/L. Do not start the next cycle until the ANC and platelet count exceed these levels.
The recommended dosage modifications during the maintenance phase are provided in Table 2. If TEMOZOLOMIDE is withheld, reduce the dose for the next cycle by 50 mg/m2 per day. Permanently discontinue TEMOZOLOMIDE in patients who are unable to tolerate a dose of 100 mg/m2 per day.
TABLE 2: Temozolomide Dosage Modifications During Maintenance Treatment
Toxicity
Interruption
Discontinuation
Absolute Neutrophil Count
Withhold TEMOZOLOMIDE if ANC less than 1 x 109/L. When ANC is above 1.5 x 109/L, resume TEMODAR at reduced dose for the next cycle.
Unable to tolerate a dose of 100 mg/m2 per day.
Platelet Count
Withhold TEMOZOLOMIDE if platelet less than 50 x 109/L. When platelet count is above 100 x 109/L, resume TEMOZOLOMIDE at reduced dose for the next cycle.
Unable to tolerate a dose of 100 mg/m2 per day.
Non-hematological Adverse Reaction (except for alopecia, nausea, vomiting)
Withhold TEMOZOLOMIDE if Grade 3 adverse reaction. When resolved to grade 1 or less, resume TEMOZOLOMIDE at reduced dose for the next cycle.
Recurrent Grade 3 after dose reduction. Grade 4 Unable to tolerate a dose of 100 mg/m2 per day.
2.2 Recommended Dosage and Dosage Modifications for Refractory Anaplastic Astrocytoma
The recommended initial dosage of TEMOZOLOMIDE is 150 mg/m2 once daily on Days 1 to 5 of each 28-day cycle. Increase the TEMOZOLOMIDE dose to 200 mg/m2 per day if the following conditions are met at the nadir and on Day 1 of the next cycle:
ANC is greater than or equal to 1.5 x 109/L and
Platelet count is greater than or equal to 100 x 109/L
Continue TEMOZOLOMIDE until disease progression or unacceptable toxicity. In the clinical trial, treatment could be continued for a maximum of 2 years, but the optimum duration of therapy is not known.
Obtain a complete blood count on Day 22 and then weekly until the ANC is above 1.5 x 109/L and the platelet count is above 100 x 109/L. Do not start the next cycle until the ANC and platelet count exceed these levels.
If the ANC is less than 1 x 109/L or the platelet count is less than 50 x 109/L during any cycle, reduce the TEMOZOLOMIDE dose for the next cycle by 50 mg/m2 per day. Permanently discontinue TEMOZOLOMIDE in patients who are unable to tolerate a dose of 100 mg/m2 per day.2.3 Preparation and Administration
TEMOZOLOMIDE is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
TEMOZOLOMIDE capsules
Administer TEMOZOLOMIDE consistently with respect to food (fasting vs. nonfasting) [see Clinical Pharmacology (12.3)]. To reduce nausea and vomiting, take TEMOZOLOMIDE on an empty stomach or at bedtime and consider antiemetic therapy prior to and/or following TEMOZOLOMIDE administration.
Swallow TEMOZOLOMIDE capsules whole. Do not open or chew capsules.
If capsules are accidentally opened or damaged, take precautions to avoid inhalation or contact with the skin or mucous membranes. In case of powder contact, the hands should be washed. -
3 DOSAGE FORMS AND STRENGTHS
· TEMOZOLOMIDE Capsules, USP for oral administration
5-mg: Capsules have opaque white bodies with opaque green caps. The capsule body is imprinted with the dosage strength.
20-mg: Capsules have opaque white bodies with opaque yellow caps. The capsule body is imprinted with the dosage strength.
100-mg: Capsules have opaque white bodies with opaque flesh caps. The capsule body is imprinted with the dosage strength.
140-mg: Capsules have opaque white bodies with transparent blue caps. The capsule body is imprinted with the dosage strength.
180-mg: Capsules have opaque white bodies with opaque orange caps. The capsule body is imprinted with the dosage strength.
250-mg: Capsules have opaque white bodies with opaque white caps. The capsule body is imprinted with the dosage strength. -
4 CONTRAINDICATIONS
TEMOZOLOMIDE is contraindicated in patients with a history of hypersensitivity reactions to:
temozolomide or any other ingredients in TEMOZOLOMIDE; and
dacarbazine, since both temozolomide and dacarbazine are metabolized to the same active metabolite 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide.
Reactions to TEMOZOLOMIDE have included anaphylaxis [see Adverse Reactions (6.2)]. -
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
Myelosuppression, including pancytopenia, leukopenia and anemia, some with fatal outcomes, have occurred with TEMOZOLOMIDE [see Adverse Reactions (6.1, 6.2)]. Geriatric patients and women have been shown in clinical trials to have a higher risk of developing myelosuppression.
Prior to dosing, patients must have an ANC of 1.5 x 109/L or greater and a platelet count of 100 x 109/L or greater.
For the concomitant phase with radiotherapy, obtain a complete blood count prior to initiation of treatment and weekly during treatment [see Dosage and Administration (2.1)].
For the 28-day treatment cycles, obtain a complete blood count prior to treatment on Day 1 and on Day 22 of each cycle. Perform complete blood counts weekly until recovery if the ANC falls below 1.5 x 109/L and the platelet count falls below 100 x 109/L [see Dosage and Administration (2.1, 2.2)].5.2 Myelodysplastic Syndrome and Secondary Malignancies
Cases of myelodysplastic syndrome and secondary malignancies, including myeloid leukemia, have been observed following TEMOZOLOMIDE administration.
5.3 Pneumocystis Pneumonia
Pneumocystis pneumonia (PCP) can occur in patients receiving TEMOZOLOMIDE. The risk of PCP is increased in patients receiving steroids or with longer treatment regimens.
For patients with newly diagnosed glioblastoma, provide PCP prophylaxis for all patients during the concomitant phase. Continue in patients who experience lymphopenia until resolution to grade 1 or less [see Dosage and Administration (2.1)].
Monitor all patients receiving TEMOZOLOMIDE for the development of lymphopenia and PCP.5.4 Hepatotoxicity
Fatal and severe hepatotoxicity have been reported in patients receiving TEMOZOLOMIDE. Perform liver tests at baseline, midway through the first cycle, prior to each subsequent cycle, and approximately two to four weeks after the last dose of TEMOZOLOMIDE.
5.5 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, TEMOZOLOMIDE can cause fetal harm when administered to a pregnant woman. Adverse developmental outcomes have been reported in both pregnant patients and pregnant partners of male patients. Oral administration of temozolomide to rats and rabbits during the period of organogenesis resulted in embryolethality and polymalformations at doses less than the maximum human dose based on body surface area.
Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment with TEMOZOLOMIDE and for at least 6 months after the final dose. Because of potential risk of genotoxic effects on sperm, advise male patients with female partners of reproductive potential to use condoms during treatment with TEMOZOLOMIDE and for at least 3 months after the final dose. Advise male patients not to donate semen during treatment with TEMOZOLOMIDE and for at least 3 months after the final dose [see Use in Specific Populations (8.1, 8.3)]. -
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
Myelosuppression [see Warnings and Precautions (5.1)].
Myelodysplastic Syndrome and Secondary Malignancies [see Warnings and Precautions (5.2)].
Pneumocystis Pneumonia [see Warnings and Precautions (5.3)].
Hepatotoxicity [see Warnings and Precautions (5.4)].6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Newly Diagnosed Glioblastoma
The safety of TEMOZOLOMIDE was evaluated in Study MK-7365-051 [see Clinical Studies (14.1)].
Forty-nine percent (49%) of patients treated with TEMOZOLOMIDE reported one or more severe or life-threatening reactions, most commonly fatigue (13%), convulsions (6%), headache (5%), and thrombocytopenia (5%).
The most common adverse reactions (≥20%) across the cumulative TEMOZOLOMIDE experience were alopecia, fatigue, nausea, and vomiting. Table 3 summarizes the adverse reactions in Newly Diagnosed Glioblastoma Trial. Overall, the pattern of reactions during the maintenance phase was consistent with the known safety profile of TEMOZOLOMIDE.
TABLE 3: Adverse Reactions (≥5%) in Patients Receiving TEMOZOLAMIDE in Newly Diagnosed Glioblastoma Trial
Adverse Reactions
Concomitant Phase
Maintenance Phase
Radiation Therapy and TEMOZOLAMIDE N=288*
Radiation Therapy Alone N=285
TEMOZOLAMIDE N=224
All Grades (%)
Grade ≥3 (%)
All Grades (%)
Grades ≥3 (%)
All Grades (%)
Grade ≥3 (%)
Skin and Subcutaneous Tissue
Alopecia
69
63
55
Rash
19
1
15
13
1
Dry Skin
2
2
5
<1
Pruritus
4
1
5
Erythema
5
5
1
General
Fatigue
54
7
49
5
61
9
Anorexia
19
1
9
<1
27
1
Headache
19
2
17
4
23
4
Weakness
3
2
3
1
7
2
Dizziness
4
1
4
5
Gastrointestinal System
Nausea
36
1
16
<1
49
1
Vomiting
20
<1
6
<1
29
2
Constipation
18
1
6
22
Diarrhea
6
3
10
1
Stomatitis
7
5
<1
9
1
Abdominal Pain
2
<1
1
5
<1
Eye
Vision Blurred
9
1
9
1
8
Injury
Radiation Injury NOS
7
4
<1
2
Central and Peripheral Nervous System
Convulsions
6
3
7
3
11
3
Memory Impairment
3
<1
4
<1
7
1
Confusion
4
1
4
2
5
2
Special Senses Other
Taste Perversion
6
2
5
Respiratory System
Coughing
5
1
1
8
<1
Dyspnea
4
2
3
1
5
<1
Psychiatric
Insomnia
5
3
<1
4
Immune System
Allergic Reaction
5
2
<1
3
Platelet, Bleeding and Clotting
Thrombocytopenia
4
3
1
8
4
Musculoskeletal System
Arthralgia
2
<1
1
6
*One patient who was randomized to radiation therapy only arm received radiation therapy and TEMOZOLAMIDE. NOS=not otherwise specified.
Note: Grade 5 (fatal) adverse reactions are included in the Grade ≥3 column.
When laboratory abnormalities and adverse reactions were combined, Grade 3 or Grade 4 neutrophil abnormalities including neutropenic reactions were observed in 8% of patients, and Grade 3 or Grade 4 platelet abnormalities including thrombocytopenic reactions, were observed in 14% of patients.
Refractory Anaplastic Astrocytoma
The safety of TEMOZOLAMIDE was evaluated in Study MK-7365-006 [see Clinical Studies (14.2)].
Myelosuppression (thrombocytopenia and neutropenia) was the dose-limiting adverse reaction. It usually occurred within the first few cycles of therapy and was not cumulative. Myelosuppression occurred late in the treatment cycle and returned to normal, on average, within 14 days of nadir counts. The median nadirs occurred at 26 days for platelets (range: 21-40 days) and 28 days for neutrophils (range: 1-44 days). Only 14% (22/158) of patients had a neutrophil nadir and 20% (32/158) of patients had a platelet nadir, which may have delayed the start of the next cycle. Less than 10% of patients required hospitalization, blood transfusion, or discontinuation of therapy due to myelosuppression.
The most common adverse reactions (≥20%) were nausea, vomiting, headache, fatigue, constipation, and convulsions.
Tables 4 and 5 summarize the adverse reactions and hematological laboratory abnormalities in Refractory Anaplastic Astrocytoma Trial.
TABLE 4: Adverse Reactions (≥5%) in Patients Receiving TEMOZOLAMIDE in Refractory Anaplastic Astrocytoma Trial
Adverse Reactions
TEMOZOLOMIDE N=158
All Reactions (%)
Grades 3-4 (%)
Gastrointestinal System
Nausea
53
10
Vomiting
42
6
Constipation
33
1
Diarrhea
16
2
Abdominal pain
9
1
Anorexia
9
1
General
Headache
41
6
Fatigue
34
4
Asthenia
13
6
Fever
13
2
Back pain
8
3
Central and Peripheral Nervous System
Convulsions
23
5
Hemiparesis
18
6
Dizziness
12
1
Coordination abnormal
11
1
Amnesia
10
4
Insomnia
10
Paresthesia
9
1
Somnolence
9
3
Paresis
8
3
Urinary incontinence
8
2
Ataxia
8
2
Dysphasia
7
1
Convulsions local
6
Gait abnormal
6
1
Confusion
5
Cardiovascular
Edema peripheral
11
1
Resistance Mechanism
Infection viral
11
Endocrine
Adrenal hypercorticism
8
Respiratory System
Upper respiratory tract infection
8
Pharyngitis
8
Sinusitis
6
Coughing
5
Skin and Appendages
Rash
8
Pruritus
8
1
Urinary System
Urinary tract infection
8
Micturition increased frequency
6
Psychiatric
Anxiety
7
1
Depression
6
Reproductive Disorders
Breast pain, female
6
Metabolic
Weight increase
5
Musculoskeletal System
Myalgia
5
Vision
Diplopia
5
Vision abnormal*
5
*This term includes blurred vision; visual deficit; vision changes; and vision troubles.
TABLE 5: Grade 3 to 4 Adverse Hematologic Laboratory Abnormalities in Refractory Anaplastic Astrocytoma Trial
TEMOZOLOMIDE *, †
Decreased lymphocytes
55%
Decreased platelets
19%
Decreased neutrophils
14%
Decreased leukocytes
11%
Decreased hemoglobin
4%
*Change from Grade 0 to 2 at baseline to Grade 3 or 4 during treatment.
†Denominator range= 142, 158
Hematological Toxicities for Advanced Gliomas:
In clinical trial experience with 110 to 111 females and 169 to 174 males (depending on measurements), females experienced higher rates of Grade 4 neutropenia (ANC < 0.5 x 109/L) and thrombocytopenia (< 20 x 109/L) than males in the first cycle of therapy (12% vs. 5% and 9% vs. 3%, respectively).
In the entire safety database for which hematologic data exist (N=932), 7% (4/61) and 9.5% (6/63) of patients > 70 years experienced Grade 4 neutropenia or thrombocytopenia in the first cycle, respectively. For patients ≤ 70 years, 7% (62/871) and 5.5% (48/879) experienced Grade 4 neutropenia or thrombocytopenia in the first cycle, respectively. Pancytopenia, leukopenia, and anemia also occurred.
Adverse reactions with TEMOZOLAMIDE for injection
Adverse reactions that were reported in 35 patients who received TEMOZOLOMIDE for injection that were not reported in patients who received TEMOZOLOMIDE capsules were pain, irritation, pruritus, warmth, swelling, and erythema at infusion site; petechiae; and hematoma.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of TEMOZOLOMIDE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the drug exposure.
Dermatologic: Toxic epidermal necrolysis and Stevens-Johnson syndrome
Immune System: Hypersensitivity reactions, including anaphylaxis. Erythema multiforme, which resolved after discontinuation of TEMOZOLOMIDE and, in some cases, recurred upon rechallenge.
Hematopoietic: Prolonged pancytopenia, which may result in aplastic anemia and fatal outcomes.
Hepatobiliary: Fatal and severe hepatotoxicity, elevation of liver enzymes, hyperbilirubinemia, cholestasis, and hepatitis.
Infections: Serious opportunistic infections, including some cases with fatal outcomes, with bacterial, viral (primary and reactivated), fungal, and protozoan organisms.
Pulmonary:Interstitial pneumonitis, pneumonitis, alveolitis, and pulmonary fibrosis.Endocrine: Diabetes insipidus
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action [see Clinical Pharmacology (12.1)] and findings from animal studies, TEMOZOLOMIDE can cause fetal harm when administered to a pregnant woman. Available postmarketing reports describe cases of spontaneous abortions and congenital malformations, including polymalformations with central nervous system, facial, cardiac, skeletal, and genitourinary system anomalies with exposure to TEMOZOLOMIDE during pregnancy. These cases report similar adverse developmental outcomes to those observed in animal studies. Administration of TEMOZOLOMIDE to rats and rabbits during the period of organogenesis caused numerous external, internal, and skeletal malformations at doses less than the maximum human dose based on body surface area (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Five consecutive days of oral administration of temozolomide at doses of 75 and 150 mg/m2 (0.38 and 0.75 times the human dose of 200 mg/m2) in rats and rabbits, respectively, during the period of organogenesis (Gestation Days 8-12) caused numerous malformations of the external and internal organs and skeleton in both species. In rabbits, temozolomide at the 150 mg/m2 dose (0.75 times the human dose of 200 mg/m2) caused embryolethality as indicated by increased resorptions.8.2 Lactation
There are no data on the presence of TEMOZOLOMIDE or its metabolites in human milk, the effects on a breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions, including myelosuppression from temozolomide in the breastfed children, advise women not to breastfeed during treatment with TEMOZOLOMIDE and for at least 1 week after the final dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating TEMOZOLOMIDE [see Use in Specific Populations (8.1)].
Contraception
Females
TEMOZOLOMIDE can cause embryo-fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with TEMOZOLOMIDE and for at least 6 months after the last dose.
Males
Because of the potential for embryofetal toxicity and genotoxic effects on sperm cells, advise male patients with pregnant partners or female partners of reproductive potential to use condoms during treatment with TEMOZOLOMIDE and for at least 3 months after the final dose [see Use in Specific Populations (8.1), Nonclinical Toxicology (13.1)].
Advise male patients not to donate semen during treatment with TEMOZOLOMIDE and for at least 3 months after the final dose.
Infertility
TEMOZOLOMIDE may impair male fertility [see Nonclinical Toxicology (13.1)]. Limited data from male patients show changes in sperm parameters during treatment with TEMOZOLOMIDE; however, no information is available on the duration or reversibility of these changes.8.4 Pediatric Use
Safety and effectiveness of TEMOZOLOMIDE have not been established in pediatric patients. Safety and effectiveness of TEMOZOLOMIDE capsules were assessed, but not established, in 2 open-label studies in pediatric patients aged 3 to18 years. In one study, 29 patients with recurrent brain stem glioma and 34 patients with recurrent high-grade astrocytoma were enrolled. In a second study conducted by the Children’s Oncology Group (COG), 122 patients were enrolled, including patients with medulloblastoma/PNET (29), high grade astrocytoma (23), low grade astrocytoma (22), brain stem glioma (16), ependymoma (14), other CNS tumors (9), and non-CNS tumors (9). The adverse reaction profile in pediatric patients was similar to adults.
8.5 Geriatric Use
In the Newly Diagnosed Glioblastoma trial, Study MK-7365-051, 15% of patients were 65 years and older. This study did not include sufficient numbers of patients aged 65 years and older to determine differences in effectiveness from younger patients. No overall differences in safety were observed between patients ≥65 years and younger patients.
In the Refractory Anaplastic Astrocytoma trial, Study MK-7365-0006, 4% of patients were 70 years and older. This study did not include sufficient numbers of patients aged 70 years and older to determine differences in effectiveness from younger patients. Patients 70 years and older had a higher incidence of Grade 4 neutropenia (25%) and Grade 4 thrombocytopenia (20%) in the first cycle of therapy than patients less than 70 years of age [see Warnings and Precautions (5.1), Adverse Reactions (6.1)].8.6 Renal Impairment
No dosage adjustment is recommended for patients with creatinine clearance (CLcr) of 36 to 130 mL/min/m2[see Clinical Pharmacology (12.3)]. The recommended dose of TEMOZOLOMIDE has not been established for patients with severe renal impairment (CLcr < 36 mL/min/m2) or for patients with end-stage renal disease on dialysis.
8.7 Hepatic Impairment
No dosage adjustment is recommended for patients with mild to moderate hepatic impairment (Child Pugh class Aand B) [see Clinical Pharmacology (12.3)]. The recommended dose of TEMOZOLOMIDE has not been established for patients with severe hepatic impairment (Child-Pugh class C).
-
10 OVERDOSAGE
Dose-limiting toxicity was myelosuppression and was reported with any dose but is expected to be more severe at higher doses. An overdose of 2000 mg per day for 5 days was taken by one patient and the adverse reactions reported were pancytopenia, pyrexia, multi-organ failure, and death. There are reports of patients who have taken more than 5 days of treatment (up to 64 days), with adverse reactions reported including myelosuppression, which in some cases was severe and prolonged, and infections and resulted in death. In the event of an overdose, monitor complete blood count and provide supportive measures as necessary.
-
11 DESCRIPTION
Temozolomide is an alkylating drug. The chemical name of temozolomide is 3,4-dihydro-3-methyl-4-oxoimidazo[5,1-d]-as-tetrazine-8-carboxamide. The structural formula of temozolomide is:
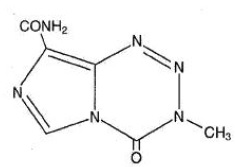
The material is a white to light tan/light pink powder with a molecular formula of C6H6N6O2 and a molecular weight of 194.15. The molecule is stable at acidic pH (<5) and labile at pH >7; hence TEMOZOLOMIDE can be administered orally and intravenously. The prodrug, temozolomide, is rapidly hydrolyzed to the active 5-(3-methyltriazen-1-yl) imidazole-4-carboxamide (MTIC) at neutral and alkaline pH values, with hydrolysis taking place even faster at alkaline pH.
TEMOZOLOMIDE Capsules, USP:
Each capsule for oral use contains either 5 mg, 20 mg, 100 mg, 140 mg, 180 mg, or 250 mg of temozolomide.
The inactive ingredients for TEMOZOLOMIDE Capsules, USP are as follows:
· TEMOZOLOMIDE 5 mg: lactose anhydrous (132.8 mg), colloidal silicon dioxide (0.2 mg), sodium starch glycolate (7.5 mg), tartaric acid (1.5 mg), and stearic acid (3.0 mg).
· TEMOZOLOMIDE 20 mg: lactose anhydrous (182.2 mg), colloidal silicon dioxide (0.2 mg), sodium starch glycolate (11 mg), tartaric acid (2.2 mg), and stearic acid (4.4 mg).
·TEMOZOLOMIDE 100 mg: lactose anhydrous (175.7 mg), colloidal silicon dioxide (0.3 mg), sodium starch glycolate (15 mg), tartaric acid (3.0 mg), and stearic acid (6.0 mg).
· TEMOZOLOMIDE 140 mg: lactose anhydrous (246 mg), colloidal silicon dioxide (0.4 mg), sodium starch glycolate (21 mg), tartaric acid (4.2 mg), and stearic acid (8.4 mg).
· TEMOZOLOMIDE 180 mg: lactose anhydrous (316.3 mg), colloidal silicon dioxide (0.5 mg), sodium starch glycolate (27 mg), tartaric acid (5.4 mg), and stearic acid (10.8 mg).
· TEMOZOLOMIDE 250 mg: lactose anhydrous (154.3 mg), colloidal silicon dioxide (0.7 mg), sodium starch glycolate (22.5 mg), tartaric acid (9.0 mg), and stearic acid (13.5 mg).
The body of the capsules is made of gelatin and titanium dioxide and is opaque white. The cap is also made of gelatin and the colors may vary based on the dosage strength. The capsule body and cap are imprinted with pharmaceutical branding ink, which contains shellac, iron oxide black, n-butyl alcohol, purified water, propylene glycol, dehydrated alcohol, isopropyl alcohol and ammonia solution.
· TEMOZOLOMIDE 5 mg: The opaque green cap contains gelatin, titanium dioxide, iron oxide yellow and FD&C Blue 2.
· TEMOZOLOMIDE 20 mg: The opaque yellow cap contains gelatin, titanium dioxide and iron oxide yellow.
· TEMOZOLOMIDE 100 mg: The opaque flesh cap contains gelatin, titanium dioxide and iron oxide red.
· TEMOZOLOMIDE 140 mg: The transparent blue cap contains gelatin, FD&C Blue #2, and titanium dioxide.
· TEMOZOLOMIDE 180 mg: The opaque orange cap contains gelatin, titanium dioxide and iron oxide red.
· TEMOZOLOMIDE 250 mg: The opaque white cap contains gelatin and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Temozolomide is not directly active but undergoes rapid nonenzymatic conversion at physiologic pH to the reactive compound 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide (MTIC). The cytotoxicity of MTIC is thought to be primarily due to alkylation of DNA. Alkylation (methylation) occurs mainly at the O6 and N7 positions of guanine.
12.3 Pharmacokinetics
Following a single oral dose of 150 mg/m2, the mean Cmax value for temozolomide was 7.5 mcg/mL and for MTIC was 282 ng/mL. The mean AUC value for temozolomide was 23.4 mcg·hr/mL and for MTIC was 864 ng·hr/mL. Following a single 90-minute intravenous infusion of 150 mg/m2, the mean Cmax value for temozolomide was 7.3 mcg/mL and for MTIC was 276 ng/mL. The mean AUC value for temozolomide was 24.6 mcg·hr/mL and for MTIC was 891 ng·hr/mL. Temozolomide exhibits linear kinetics over the therapeutic dosing range of 75 mg/m2/day to 250 mg/m2/day.
Absorption
The median Tmax is 1 hour.
Effect of Food
The mean Cmax and AUC decreased by 32% and 9%, respectively, and median Tmax increased by 2-fold (from 1-2.25 hours) when TEMOZOLOMIDE capsules were administered after a modified high-fat breakfast (587 calories comprised of 1 fried egg, 2 strips of bacon, 2 slices of toast, 2 pats of butter, and 8 oz whole milk).Distribution
Temozolomide has a mean apparent volume of distribution of 0.4 L/kg (%CV = 13%). The mean percent bound of drug-related total radioactivity is 15%.Elimination
Clearance of temozolomide is about 5.5 L/hr/m2 and the mean elimination half-life is 1.8 hours.
Metabolism
Temozolomide is spontaneously hydrolyzed at physiologic pH to the active species, MTIC and to temozolomide acid metabolite. MTIC is further hydrolyzed to 5-amino-imidazole-4-carboxamide (AIC), which is known to be an intermediate in purine and nucleic acid biosynthesis, and to methylhydrazine, which is believed to be the active alkylating species. Cytochrome P450 enzymes play only a minor role in the metabolism of temozolomide and MTIC. Relative to the AUC of temozolomide, the exposure to MTIC and AIC is 2.4% and 23%, respectively.
Excretion
About 38% of the administered temozolomide total radioactive dose is recovered over 7 days: 38% in urine and 0.8% in feces. The majority of the recovery of radioactivity in urine is unchanged temozolomide (6%), AIC (12%), temozolomide acid metabolite (2.3%), and unidentified polar metabolite(s) (17%).
Specific Populations
No clinically meaningful differences in the pharmacokinetics of temozolomide were observed based on age (range: 19-78 years), gender, smoking status (smoker vs. non-smoker), creatinine clearance (CLcr) of 36 to 130 mL/min/m2, or mild to moderate hepatic impairment (Child Pugh class A and B). The pharmacokinetics of temozolomide has not been studied in patients with CLcr < 36 mL/min/m2, end-stage renal disease on dialysis, or severe hepatic impairment (Child-Pugh class C).
Drug Interaction Studies
Effect of Other Drugs on Temozolomide Pharmacokinetics
In a multiple-dose study, administration of TEMOZOLOMIDE Capsules with ranitidine did not change the Cmax or AUC values for temozolomide or MTIC.
A population analysis indicated that administration of valproic acid decreases the clearance of temozolomide by about 5%.
A population analysis did not demonstrate any influence of coadministered dexamethasone, prochlorperazine, phenytoin, carbamazepine, ondansetron, histamine-2-receptor antagonists, or phenobarbital on the clearance of orally administered temozolomide. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Temozolomide is carcinogenic in rats at doses less than the maximum recommended human dose. Temozolomide induced mammary carcinomas in both males and females at doses 0.13 to 0.63 times the maximum human dose (25 - 125 mg/ m2) when administered orally on 5 consecutive days every 28-days for 6 cycles. Temozolomide also induced fibrosarcomas of the heart, eye, seminal vesicles, salivary glands, abdominal cavity, uterus, and prostate, carcinomas of the seminal vesicles, schwannomas of the heart, optic nerve, and harderian gland, and adenomas of the skin, lung, pituitary, and thyroid at doses 0.5 times the maximum daily dose. Mammary tumors were also induced following 3 cycles of temozolomide at the maximum recommended daily dose.
Temozolomide is a mutagen and a clastogen. In a reverse bacterial mutagenesis assay (Ames assay), temozolomide increased revertant frequency in the absence and presence of metabolic activation. Temozolomide was clastogenic in human lymphocytes in the presence and absence of metabolic activation.
Temozolomide impairs male fertility. Temozolomide caused syncytial cells/immature sperm formation at doses of 50 and 125 mg/m2 (0.25 and 0.63 times the human dose of 200 mg/m2) in rats and dogs, respectively, and testicular atrophy in dogs at 125 mg/m2.
13.2 Animal Toxicology and/or Pharmacology
Toxicology studies in rats and dogs identified a low incidence of hemorrhage, degeneration, and necrosis of the retina at temozolomide doses equal to or greater than 125 mg/m2 (0.63 times the human dose of 200 mg/m2). These changes were most commonly seen at doses where mortality was observed.
-
14 CLINICAL STUDIES
14.1 Newly Diagnosed Glioblastoma
The efficacy of TEMOZOLOMIDE was evaluated in Study MK-7365-051, a randomized (1:1), multicenter, open-label trial. Eligible patients were required to have newly diagnosed glioblastoma. Patients were randomized to receive either radiation therapy alone or concomitant TEMOZOLOMIDE 75 mg/m2 once daily starting the first day of radiation therapy and continuing until the last day of radiation therapy for 42 days (with a maximum of 49 days), followed by TEMOZOLOMIDE 150 mg/m2 or 200 mg/m2 once daily on Days 1 to 5 of each 28-day cycle, starting 4 weeks after the end of radiation therapy and continuing for 6 cycles. In both arms, focal radiation therapy was delivered as 60 Gy/30 fractions and included radiation to the tumor bed or resection site with a 2- to 3-cm margin. PCP prophylaxis was required during the concomitant phase, regardless of lymphocyte count and continued until recovery of lymphocyte count to grade 1 or less. The major efficacy outcome measure was overall survival.
A total of 573 patients were randomized, 287 to TEMOZOLOMIDE and radiation therapy and 286 to radiation therapy alone. At the time of disease progression, TEMOZOLOMIDE was administered as salvage therapy in 161 patients of the 282 (57%) in the radiation therapy alone arm and 62 patients of the 277 (22%) in the TEMOZOLOMIDE and radiation therapy arm.
The addition of concomitant and maintenance TEMOZOLOMIDE to radiation therapy for the treatment of patients with newly diagnosed glioblastoma showed a statistically significant improvement in overall survival compared to radiotherapy alone (Figure 1). The hazard ratio (HR) for overall survival was 0.63 (95% CI: 0.52, 0.75) with a log-rank P<0.0001 in favor of the TEMOZOLOMIDE arm. The median survival was increased by 2.5 months in the TEMOZOLOMIDE arm.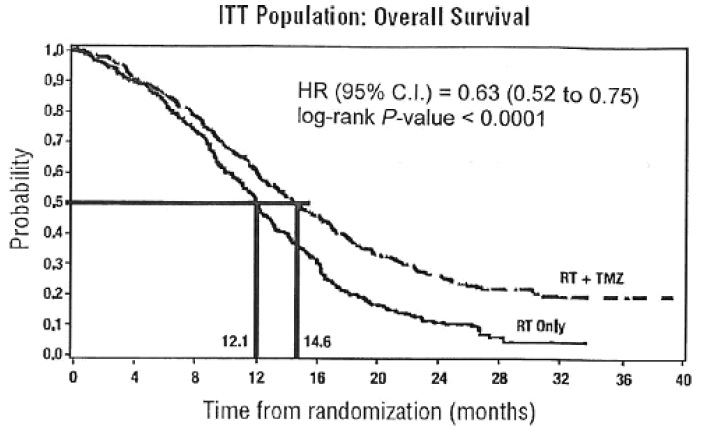
FIGURE 1: Kaplan-Meier Curves for Overall Survival (ITT Population) in Newly Diagnosed Glioblastoma Trial
14.2 Refractory Anaplastic Astrocytoma
The efficacy of TEMOZOLOMIDE was evaluated in Study MK-7365-006, a single-arm, multicenter trial. Eligible patients had anaplastic astrocytoma at first relapse and a baseline Karnofsky performance status (KPS) of 70 or greater. Patients had previously received radiation therapy and may also have previously received a nitrosourea with or without other chemotherapy. Fifty-four patients had disease progression on prior therapy with both a nitrosourea and procarbazine and their malignancy was considered refractory to chemotherapy (refractory anaplastic astrocytoma population). TEMOZOLOMIDE capsules were given on Days 1 to 5 of each 28-day cycle at a starting dose of 150 mg/m2/day. If ANC was ≥1.5 x 109/L and
platelet count was ≥100 x 109/L at the nadir and on Day 1 of the next cycle, the TEMOZOLOMIDE dose was increased to 200 mg/m2/day. The major efficacy outcome measure was progression-free survival at 6 months and the additional efficacy outcome measures were overall survival and overall response rate.
In the refractory anaplastic astrocytoma population (n=54), the median age was 42 years (range: 19 to 76); 65% were male; and 72% had a KPS of >80. Sixty-three percent of patients had surgery other than a biopsy at the time of initial diagnosis. Of those patients undergoing resection, 73% underwent a subtotal resection and 27% underwent a gross total resection. Eighteen percent of patients had surgery at the time of first relapse. The median time from initial diagnosis to first relapse was 13.8 months (range: 4.2 months to 6.3 years).In the refractory anaplastic astrocytoma population, the overall response rate (CR + PR) was 22% (12 of 54 patients) and the complete response rate was 9% (5 of 54 patients). The median duration of all responses was 50 weeks (range: 16 to 114 weeks) and the median duration of complete responses was 64 weeks (range: 52 to 114 weeks). In this population, progression-free survival at 6 months was 45% (95% CI: 31%, 58%) and progression-free survival at 12 months was 29% (95% CI: 16%, 42%). Median progression-free survival was 4.4 months. Overall survival at 6 months was 74% (95% CI: 62%, 86%) and 12-month overall survival was 65% (95% CI: 52%, 78%). Median overall survival was 15.9 months.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
TEMOZOLOMIDE Capsules, USP are supplied in amber glass bottles, with child-resistant polypropylene caps (not supplied in sachets) containing the following capsule strengths:
TEMOZOLOMIDE CapsulesUSP, 5 mg:
Have opaque white bodies with opaque green caps. The capsule body is imprinted with the dosage strength.
They are supplied as follows: Bottles: 5-count – NDC: 67877-537-07
14-count – NDC: 67877-537-14
TEMOZOLOMIDE CapsulesUSP, 20 mg:
Have opaque white bodies with opaque yellow caps. The capsule body is imprinted with the dosage strength.
They are supplied as follows: Bottles: 5-count – NDC: 67877-538-07
14-count – NDC: 67877-538-14
TEMOZOLOMIDE CapsulesUSP, 100 mg:
Have opaque white bodies with opaque flesh caps. The capsule body is imprinted with the dosage strength.
They are supplied as follows: Bottles: 5-count – NDC: 67877-539-07
14-count – NDC: 67877-539-14
TEMOZOLOMIDE CapsulesUSP, 140 mg:
Have opaque white bodies with transparent blue caps. The capsule body is imprinted with the dosage strength.
They are supplied as follows: Bottles: 5-count – NDC: 67877-540-07
14-count – NDC: 67877-540-14
TEMOZOLOMIDE CapsulesUSP 180 mg:
Have opaque white bodies with opaque orange caps. The capsule body is imprinted with the dosage strength.
They are supplied as follows: Bottles: 5-count – NDC: 67877-541-07
14-count – NDC: 67877-541-14
TEMOZOLOMIDE CapsulesUSP 250 mg:
Have opaque white bodies with opaque white caps. The capsule body is imprinted with the dosage strength.
They are supplied as follows: Bottles:
5-count – NDC: 67877-542-07
Store TEMOZOLOMIDE Capsules, USP at 25°C (77°F); excursions permitted to 15°–30°C (59°–86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Myelosuppression
Inform patients that TEMOZOLOMIDE can cause low blood cell counts and the need for frequent monitoring of blood cell counts. Advise patients to contact their healthcare provider immediately for bleeding, fever, or other signs of infection [see Warnings and Precautions (5.1)].
Myelodysplastic Syndrome and Secondary Malignancies
Advise patients of the increased risk of myelodysplastic syndrome and secondary malignancies [see Warnings and Precautions (5.2)].
Pneumocystis Pneumonia
Advise patients of the increased risk of Pneumocystis pneumonia and to contact their healthcare provided immediately for new or worsening pulmonary symptoms. Inform patients that prophylaxis for Pneumocystis pneumonia may be needed [see Dosage and Administration (2.1), Warnings and Precautions (5.3)].
Hepatotoxicity
Advise patients of the increased risk of hepatotoxicity and to contact their healthcare provider immediately for signs or symptoms of hepatoxicity [see Warnings and Precautions (5.4)].
Administration Instructions
Advise patient to not open capsules. If capsules are accidentally opened or damaged, advise patients to take rigorous precautions with capsule contents to avoid inhalation or contact with the skin or mucous membranes. In case of powder contact, the hands should be washed. [see Dosage and Administration (2.3)].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.5), Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with TEMOZOLOMIDE and for at least 6 months after the last dose [see Use in Specific Populations (8.3)].
Advise male patients with pregnant partners or female partners of reproductive potential to use condoms during treatment with TEMOZOLOMIDE and for at least 3 months after the final dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Advise male patients not to donate semen during treatment with TEMOZOLOMIDE and for at least 3 months after the final dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Lactation
Advise women not to breastfeed during treatment with TEMOZOLOMIDE and for at least 1 week after the final dose [see Use in Specific Populations (8.2)].
Infertility
Advise males of reproductive potential that TEMOZOLOMIDE may impair fertility [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].Manufactured by:
Deva Holding A.S.
1 Merkez Mahallesi
Istanbul (Turkey), 34303
Distributed by:
Ascend Laboratories, LLC
339 Jefferson Road
Parsippany, NJ 07054
Revised: 12/2019
-
Patient Information
TEMOZOLOMIDE (TEM-oh-ZOL-oh-mide) Capsules, USP
What is the most important information I should know about TEMOZOLOMIDE?
TEMOZOLOMIDE may cause birth defects.
Females and female partners of male patients who take TEMOZOLOMIDE:
o Avoid becoming pregnant while taking TEMOZOLOMIDE.
o Females who can become pregnant should use an effective form of birth control (contraception) during treatment and for at least 6 months after your last dose of TEMOZOLOMIDE. Your doctor should to do a pregnancy test to make sure that you are not pregnant before you start taking TEMOZOLOMIDE.
o Tell your doctor right away if you become pregnant or think you are pregnant during treatment with TEMOZOLOMIDE.
Males taking TEMOZOLOMIDE and have a female partner who is pregnant or who can become pregnant:
o Use a condom for birth control (contraception) during treatment and for at least 3 months after taking your final dose of TEMOZOLOMIDE.
o Do not donate semen during treatment and for at least 3 months after your final dose of TEMOZOLOMIDE.
See the section "What are the possible side effects of TEMOZOLOMIDE?" for more information about side effects.
What is TEMOZOLOMIDE?
TEMOZOLOMIDE is a prescription medicine used to treat adults with certain brain cancer tumors. It is not known if TEMOZOLOMIDE is safe and effective in children.
Who should not take TEMOZOLOMIDE?
Do not take TEMOZOLOMIDE if you:
have had an allergic reaction to temozolomide or any of the other ingredients in TEMOZOLOMIDE. See the end of this leaflet for a list of ingredients in TEMOZOLOMIDE. Symptoms of an allergic reaction with TEMOZOLOMIDE may include: a red itchy rash, or a severe allergic reaction, such as trouble breathing, swelling of the face, throat, or tongue, or severe skin reaction. If you are not sure, ask your doctor.
have had an allergic reaction to dacarbazine (DTIC), another cancer medicine.
What should I tell my doctor before taking TEMOZOLOMIDE?
Tell your doctor about all your medical conditions, including if you:
have kidney problems
have liver problems
are pregnant or plan to become pregnant. See “What is the most important information I should know about TEMOZOLOMIDE?”
are breast-feeding or plan to breastfeed. It is not known if TEMOZOLOMIDE passes into your breast milk. Do not breastfeed during treatment and for at least 1 week after your last dose of TEMOZOLOMIDE.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Especially tell your doctor if you take a medicine that contains valproic acid
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How should I take TEMOZOLOMIDE?
TEMOZOLOMIDE may be taken 2 different ways:
o you may take TEMOZOLOMIDE by mouth as a capsule, or
o you may receive TEMOZOLOMIDE as an intravenous (IV) injection into your vein. Your doctor will decide the best way for you to take TEMOZOLOMIDE. Take TEMOZOLOMIDE exactly as prescribed by your doctor.
There are 2 common dosing schedules for taking TEMOZOLOMIDE depending on the type of brain cancer tumor that you have.
People with certain brain can cancer tumors take or receive TEMOZOLOMIDE:
o 1 time each day for 42 days in a row (possibly 49 days depending on side effects) along with receiving radiation treatment. This is 1 cycle of treatment.
o After this, your doctor may prescribe 6 more cycles of TEMOZOLOMIDE as “maintenance” treatment. For each of these cycles, you take or receive TEMOZOLOMIDE 1 time each day for 5 days in a row and then you stop taking it for the next 23 days. This is a 28-day maintenance treatment cycle.
People with certain other brain cancer tumors take or receive TEMOZOLOMIDE:
o 1 time each day for 5 days in a row only, and then stop taking it for the next 23 days. This is 1 cycle of treatment (28 days).
o Your doctor will watch your progress on TEMOZOLOMIDE and decide how long you should take it. You might take TEMOZOLOMIDE until your tumor gets worse or for possibly up to 2 years.
If your doctor prescribes a treatment regimen that is different from the information in this leaflet, make sure you follow the instructions given to you by your doctor.
Your doctor may change your dose of TEMOZOLOMIDE, or tell you to stop TEMOZOLOMIDE for a short period of time or permanently if you have certain side effects.
Your doctor will decide how many treatment cycles of TEMOZOLOMIDE that you will receive, depending on how you respond to and tolerate treatment.
TEMOZOLOMIDE Capsules, USP:
Take TEMOZOLOMIDE Capsules, USP exactly as your doctor tells you to.
TEMOZOLOMIDE capsules contain a white capsule body with a color cap and the colors vary based on the dosage strength. Your doctor may prescribe more than 1 strength of TEMOZOLOMIDE capsules, USP for you, so it is important that you understand how to take your medicine the right way. Be sure that you understand exactly how many capsules you need to take on each day of your treatment, and what strengths to take. This may be different whenever you start a new cycle.
Do not take more TEMOZOLOMIDE than prescribed.
Talk to your doctor or pharmacist before taking your dose if you are not sure how much TEMOZOLOMIDE to take. This will help to prevent taking too much TEMOZOLOMIDE and decrease your chances of getting serious side effects.
Take each day’s dose of TEMOZOLOMIDE Capsules, USP at one time, with a full glass of water.
Swallow TEMOZOLOMIDE Capsules, USP whole. Do not chew, open, or split the capsules.
Take TEMOZOLOMIDE capsules, USP at the same time each day.
Take TEMOZOLOMIDE the same way each time, either with food or without food.
If TEMOZOLOMIDE Capsules, USP are accidentally opened or damaged, be careful not to breathe in (inhale) the powder from the capsules or get the powder on your skin or mucous membranes (for example, in your nose or mouth). If contact with any of these areas happens, flush the area with water.
To help reduce nausea and vomiting, try to take TEMOZOLOMIDE on an empty stomach or at bedtime. Your doctor may prescribe medicine to help prevent or treat nausea, or other medicines to reduce side effects with TEMOZOLOMIDE .
See your doctor regularly to check your progress. Your doctor will check you for side effects.
If you take more TEMOZOLOMIDE than prescribed, call your doctor or get emergency medical help right away.
What are the possible side effects of TEMOZOLOMIDE?
TEMOZOLOMIDE can cause serious side effects, including:
See “What is the most important information I should know about TEMOZOLOMIDE?”
Decreased blood cell counts.
TEMOZOLOMIDE can affect your bone marrow and cause you to have decreased blood cell counts. Decreased white blood cell count, red blood cell count and platelet count are common with TEMOZOLOMIDE but it can also be severe and lead to death.
o Your doctor will do blood tests regularly to check your blood cell counts before you start and during treatment with TEMOZOLOMIDE.
o Your doctor may need to change the dose of TEMOZOLOMIDE, or when you get it depending on your blood cell counts.
o People who are age 70 or older and women have a higher risk for developing decreased blood cell counts during treatment with TEMOZOLOMIDE.
Secondary cancers. Blood problems such as myelodysplastic syndrome (MDS) and new cancers (secondary cancers), including a certain kind of leukemia, can happen in people who take TEMOZOLOMIDE. Your doctor will monitor you for this.
Pneumocystis pneumonia (PCP). PCP is an infection that people can get when their immune system is weak. TEMOZOLOMIDE decreases white blood cells, which makes your immune system weaker and can increase your risk of getting PCP.
o People who are taking steroid medicines or who stay on TEMOZOLOMIDE for a longer period of time may have an increased risk of getting PCP infection.
o Anyone who takes TEMOZOLOMIDE will be watched carefully by their doctor for low blood cell counts and this infection.
o Tell your doctor if you have any of the following signs and symptoms of PCP infection: shortness of breath, or fever, chills, dry cough.
Liver problems.Liver problems can happen with TEMOZOLOMIDE and can sometimes be severe and lead to death. Your doctor will do blood tests to check your liver function before you start taking TEMOZOLOMIDE , during treatment, and about 2 to 4 weeks after your last dose of TEMOZOLOMIDE .
Common side effects with TEMOZOLOMIDE include:
hair loss
feeling tired
nausea and vomiting.
headache
constipation
loss of appetite
convulsions
rash
diarrhea
unable to move (paralysis) on one side of the body
weakness
fever
dizziness
coordination problems
viral infection
memory loss
sleep problems
Additional side effects seen with TEMOZOLOMIDE for injection:
pain, irritation, itching, warmth, swelling or redness at the site of infusion
bruising or small red or purple spots under the skin
TEMOZOLOMIDE can affect fertility in males and may affect your ability to father a child. Talk with your doctor if fertility is a concern for you.
Tell your doctor about any side effect that bothers you or that does not go away.
These are not all the possible side effects with TEMOZOLOMIDE. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store TEMOZOLOMIDE Capsules, USP?
Store TEMOZOLOMIDE Capsules, USP at 77°F (controlled room temperature). Storage at 59°F to 86°F (15°C to 30°C) is permitted occasionally.
Keep TEMOZOLOMIDE Capsules, USP out of the reach of children and pets.
General information about TEMOZOLOMIDE.
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use TEMOZOLOMIDE for a condition for which it was not prescribed. Do not give TEMOZOLOMIDE to other people, even if they have the same symptoms that you have. It may harm them.
This leaflet summarizes the most important information about TEMOZOLOMIDE. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about TEMOZOLOMIDE that is written for health professionals.
For more information, contact Ascend Laboratories, LLC at 1-877-ASC-RX01 (877-272-7901).
What are the ingredients in TEMOZOLOMIDE?
TEMOZOLOMIDE Capsules, USP:
Active ingredient: temozolomide.
Inactive ingredients: lactose anhydrous, sodium starch glycolate, colloidal silicon dioxide, tartaric acid, and stearic acid.
The body of the capsules is made of gelatin and titanium dioxide and is opaque white. The cap is also made of gelatin and the colors vary based on the dosage strength. The capsule body and cap are imprinted with pharmaceutical branding ink, which contains shellac, iron oxide black, n-butyl alcohol, purified water, propylene glycol, dehydrated ethanol, isopropyl alcohol and ammonium hydroxide.
TEMOZOLOMIDE 5 mg: The opaque green cap contains gelatin, titanium dioxide, iron oxide yellow and FD&C Blue #2.
TEMOZOLOMIDE 20 mg: The opaque yellow cap contains gelatin, titanium dioxide and iron oxide yellow.
TEMOZOLOMIDE 100 mg: The opaque flesh cap contains gelatin, titanium dioxide and iron oxide red.
TEMOZOLOMIDE 140 mg: The transparent blue cap contains gelatin and FD&C Blue #2
TEMOZOLOMIDE 180 mg: The opaque orange cap contains gelatin, titanium dioxide and iron oxide red.
TEMOZOLOMIDE 250 mg: The opaque white cap contains gelatin and titanium dioxide.
Manufactured by:
Deva Holding A.S.
1Merkez Mahallesi
Istanbul (Turkey), 34303

Distributed by:
Ascend Laboratories, LLC
339 Jefferson Road
Parsippany, NJ 07054

Revised: 12/2019
- PACKAGE LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TEMOZOLOMIDE
temozolomide capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67877-537 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TEMOZOLOMIDE (UNII: YF1K15M17Y) (TEMOZOLOMIDE - UNII:YF1K15M17Y) TEMOZOLOMIDE 5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TARTARIC ACID (UNII: W4888I119H) STEARIC ACID (UNII: 4ELV7Z65AP) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color WHITE (opaque white bodies) , GREEN (opaque green caps) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code 5mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67877-537-07 5 in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2017 2 NDC: 67877-537-14 14 in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207658 04/28/2017 TEMOZOLOMIDE
temozolomide capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67877-538 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TEMOZOLOMIDE (UNII: YF1K15M17Y) (TEMOZOLOMIDE - UNII:YF1K15M17Y) TEMOZOLOMIDE 20 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TARTARIC ACID (UNII: W4888I119H) STEARIC ACID (UNII: 4ELV7Z65AP) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color WHITE (opaque white bodies) , YELLOW (opaque yellow caps) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code 20mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67877-538-07 5 in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2017 2 NDC: 67877-538-14 14 in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207658 04/28/2017 TEMOZOLOMIDE
temozolomide capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67877-539 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TEMOZOLOMIDE (UNII: YF1K15M17Y) (TEMOZOLOMIDE - UNII:YF1K15M17Y) TEMOZOLOMIDE 100 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TARTARIC ACID (UNII: W4888I119H) STEARIC ACID (UNII: 4ELV7Z65AP) GELATIN (UNII: 2G86QN327L) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (opaque white bodies) , YELLOW (opaque yellow caps) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code 100mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67877-539-07 5 in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2017 2 NDC: 67877-539-14 14 in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207658 04/28/2017 TEMOZOLOMIDE
temozolomide capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67877-540 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TEMOZOLOMIDE (UNII: YF1K15M17Y) (TEMOZOLOMIDE - UNII:YF1K15M17Y) TEMOZOLOMIDE 140 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TARTARIC ACID (UNII: W4888I119H) STEARIC ACID (UNII: 4ELV7Z65AP) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color WHITE (opaque white bodies) , BLUE (transparent blue caps) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code 140mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67877-540-07 5 in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2017 2 NDC: 67877-540-14 14 in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207658 04/28/2017 TEMOZOLOMIDE
temozolomide capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67877-541 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TEMOZOLOMIDE (UNII: YF1K15M17Y) (TEMOZOLOMIDE - UNII:YF1K15M17Y) TEMOZOLOMIDE 180 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TARTARIC ACID (UNII: W4888I119H) STEARIC ACID (UNII: 4ELV7Z65AP) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color WHITE (opaque white bodies) , ORANGE (opaque orange caps) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code 180mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67877-541-07 5 in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2017 2 NDC: 67877-541-14 14 in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207658 04/28/2017 TEMOZOLOMIDE
temozolomide capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67877-542 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TEMOZOLOMIDE (UNII: YF1K15M17Y) (TEMOZOLOMIDE - UNII:YF1K15M17Y) TEMOZOLOMIDE 250 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TARTARIC ACID (UNII: W4888I119H) STEARIC ACID (UNII: 4ELV7Z65AP) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (opaque white bodies) , WHITE (opaque white caps) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code 250mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67877-542-07 5 in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207658 04/28/2017 Labeler - Ascend Laboratories, LLC (141250469) Establishment Name Address ID/FEI Business Operations Deva Holding A.S 502664395 MANUFACTURE(67877-537, 67877-538, 67877-539, 67877-540, 67877-541, 67877-542)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.





