NOBATOLﺡ؟ (phenobarbital) tablets CIV
Nobatol by
Drug Labeling and Warnings
Nobatol by is a Animal medication manufactured, distributed, or labeled by MWI/VetOne. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NOBATOL- phenobarbitalﺡ tabletﺡ
MWI/VetOne
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
NOBATOLﺡ؟ (phenobarbital) tablets CIV
DESCRIPTION
Phenobarbital is a barbituric acid derivative for oral administration and occurs as a white, odorless, slightly bitter powder that is soluble in chloroform, freely soluble in alcohol or ether, and slightly soluble in water. Its saturated solution has a pH of about 5.6. Chemically, it is 5-ethyl-5-phenylbarbituric acid with the molecular formula C12H12N2O3 (232.24). The structural formula is as follows:
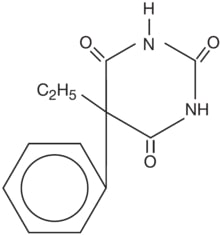
NOBATOLﺡ؟ is a round tablet, brown in color and contains phenobarbital formulated together with meat components.
CLINICAL PHARMACOLOGY
After oral administration of phenobarbital to dogs, the drug is rapidly absorbed with peak plasma concentrations occurring between 4-8 hours after administration. Bioavailability is reported to range between 70%-90%. Approximately 40%-60% of the plasma concentration is protein bound. Metabolism occurs primarily in the liver by hydroxylated oxidation of the phenyl group in the para position, and about 25% of the drug is excreted unchanged in the urine. Elimination half-lives vary considerably between individuals and range from about 12-125 hours. Steady state serum concentrations are typically not reached until 1-2 weeks after treatment is initiated.
Phenobarbital is a well-known inducer of hepatic microsomal P-450 enzymes. Elevations in hepatic enzymes can occur without hepatic disease.
INDICATIONS AND USAGE
NOBATOLﺡ؟ is indicated for the prevention of seizures due to generalized epilepsy in dogs.
Extra-label use is prohibited.
DOSAGE AND ADMINISTRATION
For oral administration. The required dosage will differ to some extent between individual dogs and with the nature and severity of the disorder.
Dosing should occur at the same time each day in a consistent manner to optimize treatment success.
For initial therapy, dogs should be given a starting dose of 2.5-3.0 mg / kg bodyweight every 12 hours.
If seizures are not being controlled, the dosage may be increased incrementally, with associated monitoring of serum phenobarbital levels. The most safe and efficacious therapeutic range reported for dogs is 15-35ﺳﺙg/ml. High plasma concentrations may be associated with hepatotoxicity. Blood samples could be taken at the same time to allow plasma phenobarbital concentration to be determined preferably during trough levels, shortly before the next dose of phenobarbital is due.
Plasma concentrations should be interpreted in conjunction with the observed response to therapy and a full clinical assessment, including monitoring, for evidence of toxic effects in each animal.
If the seizure control is inadequate, care should be taken when increasing the dose as toxic levels may be reached or exceeded. Peak and trough plasma concentrations of phenobarbital may need to be measured in such animals.
Tablets can be divided into equal halves to ensure accurate dosing.
To break a tablet into two halves, place the tablet on an even surface with the scored side up, hold one half of the tablet and press down on the other half.
CONTRAINDICATIONS
Do not use in dogs with seriously impaired hepatic function, serious renal or cardiovascular disorders. Use cautiously in dogs that are hypovolemic, anemic, or have respiratory depression.
Do not use in case of hypersensitivity to the active substance or to any other barbiturates or to any of the inactive ingredients.
WARNINGS / PRECAUTIONS
The decision to start antiepileptic drug therapy with phenobarbital should be evaluated for each individual case and depends on number, frequency, duration, and severity of seizures in dogs.
Do not confuse phenobarbital with pentobarbital.
This product is not intended to be used in animals intended for use as food for humans or food-producing animals.
Warning: May be habit forming.
Caution is recommended in dogs with impaired hepatic and renal function, hypovolemia, anemia and cardiac or respiratory dysfunction. The chance of hepatotoxic side effects can be diminished or delayed using an effective dose that is as low as possible. Monitoring of hepatic parameters is recommended in case of a prolonged therapy.
It is recommended to assess the clinical pathology of the dog 2-3 weeks after start of treatment and afterwards every 4-6 months, e.g. measurement of hepatic enzymes and serum bile acids. It is important to know that the effects of hypoxia, etc. do cause increased levels of hepatic enzymes after a seizure. Phenobarbital may increase the activity of serum alkaline phosphatase and transaminases. These may demonstrate non-pathological changes, but could also represent hepatotoxicity. Liver function tests are recommended. Increased liver enzyme values may not always require a dose reduction of phenobarbital if the serum bile acids are in the normal range.
The tablets are flavored. In order to avoid any accidental ingestion, store tablets in original container and out of reach of children and animals.
Special precautions to be taken by the person administering the veterinary medicinal product to dogs:
- Barbiturates can cause hypersensitivity. People with known hypersensitivity to barbiturates should avoid contact with the product.
- Accidental ingestion may cause intoxication and could be fatal, particularly for children. Take utmost care that children do not come in contact with the product.
- Phenobarbital is teratogenic and may be toxic to unborn and breastfeeding children; it may affect the developing brain and lead to cognitive disorders. Phenobarbital is excreted in breast milk. Pregnant women, women of childbearing age, and women who are breastfeeding should avoid accidental ingestion and prolonged skin contact with the product.
- Keep this product in its original packaging to help avoid accidental ingestion.
It is advisable to wear disposable gloves during administration of the product to reduce skin contact. In case of accidental ingestion, seek medical attention immediately, advising medical services of barbiturate poisoning; show the package insert or the label to the physician. If possible, the physician should be informed about the time and amount of ingestion, as this information may help to ensure that appropriate treatment is given. Wash hands thoroughly after use.
DRUG INTERACTIONS
A therapeutic dose of phenobarbital for antiepileptic therapy can significantly induce plasma proteins, (such as ﺳﺎ1- acid glycoprotein, AGP), which bind drugs. Phenobarbital may reduce the activity of some drugs by increasing the rate of metabolism through induction of drug metabolizing enzymes in liver microsomes. Therefore, special attention must be paid to the pharmacokinetics and doses of drugs simultaneously administered. The plasmatic concentration of a range of drugs (for example cyclosporine, thyroid hormones, and theophylline) is decreased in the case of concurrent administration of phenobarbital. Concurrent use with other drugs having a central depressive action (like narcotic analgesics, morphine derivatives, phenothiazines, antihistamines, clomipramine, and chloramphenicol) can increase the effect of phenobarbital.
Cimetidine and ketoconazole are inhibitors of hepatic enzymes: concurrent use with phenobarbital can induce an increase of serum concentration of phenobarbital. Phenobarbital may decrease the absorption of griseofulvin. Concurrent use with potassium bromide increases the risk of pancreatitis. Use of phenobarbital tablets in conjunction with primidone is not recommended as primidone is predominantly metabolized to phenobarbital.
The following drugs can decrease the convulsive threshold: quinolones, high doses of ﺳﺎ-lactam antibiotic, theophylline, aminophylline, cyclosporine and propofol for example. Medications which may alter the seizure threshold should only be used if really necessary and when no safer alternative exists.
REPRODUCTIVE / PREGNANCY / NURSING SAFETY
Phenobarbital crosses the placental barrier and at higher doses (reversible) withdrawal symptoms in newborns cannot be excluded. Studies in laboratory animals have shown evidence of action of phenobarbital on prenatal growth, especially concerning sexual development. Neonatal bleeding tendencies have been associated with phenobarbital treatment during pregnancy. Administration of Vitamin K to the dam for 10 days before parturition may help to minimize these effects on the fetus.
The safety of the product has not been established during pregnancy of dogs. The benefits of treatment may be greater than the potential risks associated with epileptic seizures on the fetus (hypoxia and acidosis). Therefore, in case of pregnancy, termination of antiepileptic treatment is not recommended; however, the dose should be as low as possible.
Phenobarbital is excreted in small amounts in breast milk and during nursing, pups should be monitored carefully for undesired sedative effects. Weaning early may be an option. If somnolence/sedative effects (that could interfere with suckling) appear in nursing newborns, an artificial suckling method should be chosen.
Use during pregnancy and lactation only according to the benefit/risk assessment by the responsible veterinarian.
ADVERSE REACTIONS
All adverse effects noted below have been reported very rarely.
During start of therapy ataxia and sedation can occur, but these effects are usually transitory and disappear in most, but not all, with continued medication. Some animals can demonstrate a paradoxical hyperexcitability, particularly after first starting therapy. As this hyperexcitability is not linked to overdosage, no reduction of dosage is needed. Polyuria, polydipsia, and polyphagia can occur at average or higher therapeutic active serum concentrations; these effects can be diminished by limiting intake of food. Sedation and ataxia often become significant concerns as serum levels reach the higher ends of the therapeutic range. High plasma concentrations may be associated with hepatotoxicity. Phenobarbital can have deleterious effects on stem cells from bone marrow. Consequences are immunotoxic pancytopenia and/or neutropenia. These reactions disappear after the treatmentﻗs withdrawal. Treating dogs with phenobarbital may lower their TT4 or FT4 serum levels, however this may not be an indication of hypothyroidism. Treatment with thyroid hormone replacement should only be started if there are clinical signs of the disease.
If adverse effects are severe, a decrease in the administered dose is recommended.
In case of accidental human ingestion, seek medical attention immediately.
In case of accidental overdose in dogs, call 1-561-570-1875.
OVERDOSAGE
Symptoms of overdose are:
- depression of the central nervous system demonstrated by signs ranging from sleep to coma,
- respiratory problems,
- cardiovascular problems, hypotension and shock leading to renal failure and death.
In case of overdose, remove ingested product from the stomach and give respiratory and cardiovascular support as necessary.
There is no specific antidote, but CNS stimulants, (like Doxapram) may stimulate the respiratory center.
STORAGE CONDITIONS
Store in a dry place at controlled room temperature, between 59 and 77ﺡﺍF (15-25ﺡﺍC). Store unused tablets in the original closed container.
Store product according to state and local requirements.
HOW SUPPLIED
NOBATOLﺡ؟ 30 mg: Brown, round, tablets scored on one side and debossed ﻗMBﻗ above the score and ﻗ30ﻗ below the score. The other side is plain. Available in bottles of 100 tablets, NDC: 86136-017-10, and 500 tablets, NDC: 86136-017-50.
NOBATOLﺡ؟ 60 mg: Brown, round, tablets scored on one side and debossed ﻗMBﻗ above the score and ﻗ60ﻗ below the score. The other side is plain. Available in bottles of 100 tablets, NDC: 86136-018-10, and 500 tablets, NDC: 86136-018-50.
REFERENCES
- Lepitil 60mg Flavoured Tablets for Dogs. Summary of Product Characteristics. Chanelle Pharmaceuticals Manufacturing Ltd; July 2018.
- Epityl 60mg Flavoured Tablets for Dogs. Summary of Product Characteristics. Chanelle Pharmaceuticals Manufacturing Ltd; June 2018.
- 2015 ACVIM Small Animal Consensus Statement on Seizure Management in Dogs. Journal of Veterinary Internal Medicine. 2016: (30):477-490
- Phenobarbital/Phenobarbital Sodium. Plumbs Veterinary Medication Handbook. 7th Edition. 2011. Pages 1081-1086
- Management of canine epilepsy with phenobarbital and potassium bromide. Clinical Pharmacology. Can Veterianry Journal. 1994: (35)
| NOBATOLﺡ
phenobarbital tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NOBATOLﺡ
phenobarbital tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler -ﺡ MWI/VetOne (019926120) |
Trademark Results [Nobatol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NOBATOL 90064864 not registered Live/Pending |
Mizner Bioscience, LLC 2020-07-21 |
ﺡ۸ 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

