Salicor by Clinic Pharma Salicor™
Salicor by
Drug Labeling and Warnings
Salicor by is a Otc medication manufactured, distributed, or labeled by Clinic Pharma. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SALICOR- triethanolamine salicylate patch
Clinic Pharma
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Salicor™
DESCRIPTION
Salicor™ is an external analgesic product containing 10% triethanolamine salicylate as the active ingredient. Triethanolamine salicylate is an organic compound formed between triethanolamine and salicylic acid, where triethanolamine neutralizes the acidity of salicylic acid. This external analgesic is designed for temporary relief of minor pain associated with arthritis, simple backache, muscle strains, sprains, and bruises. Unlike other external analgesics, triethanolamine salicylate has no distinct odor, which improves patient acceptability.
Triethanolamine Salicylate 10% Rx only
Inactive ingredients: crospovodone, polyisobutylene rubber PSA adhesive
Pharmacodynamics
Triethanolamine salicylate is an external analgesic that functions by inhibiting cyclooxygenase (COX) enzymes, which are involved in the production of pro-inflammatory factors such as prostaglandins and thromboxanes. These enzymes play a crucial role in generating pain and inflammation in conditions like arthritis, muscle strains, and sprains. While salicylates, such as aspirin, are known to inhibit COX enzymes, the specific mechanism of triethanolamine salicylate in topical applications may differ slightly. It is generally believed to act similarly to topical NSAIDs, reducing inflammation and pain locally. However, the evidence regarding its selectivity towards COX-2 enzymes is less clear.
Absorption
Following external administration of Salicor™ to healthy volunteers, no detectable levels of salicylic acid were found in the serum, indicating low systemic absorption. This minimal absorption is advantageous as it reduces the risk of systemic side effects commonly associated with oral salicylates.
Studies have shown that urinary recovery of total salicylate during the first 24 hours after external application was only 6.9 mg, which represents approximately 1.4% of the total dose applied.
Distribution
Triethanolamine salicylate is transported and distributed within cells and tissues through various mechanisms. Studies in canines and humans have shown that transdermal absorption of salicylate from triethanolamine salicylate preparations applied to skin is consistent and reproducible, with measurable tissue salicylate levels at the application site.
The distribution appears to be
concentration-dependent, with tissue salicylate levels directly proportional to the concentrations of the active ingredient in the formulation up to the 10% preparation.
Metabolism
While specific metabolism data for externally applied Salicor™ is limited due to its low systemic absorption, the small amount that does enter the systemic circulation is likely metabolized similarly to other salicylates. The primary metabolic pathways include:
- Conjugation with glycine to form salicyluric acid (approximately 75%).
- Conjugation with glucuronic acid to produce salicyl acyl and phenolic glucuronides (about 15%).
- Oxidation to gentisic acid and other hydroxylated derivatives (minor pathway).
- Free, unmodified salicylate accounts for 10–30% of excreted metabolites, depending on dosage and individual factors like urinary pH. Metabolism primarily occurs in the liver, with some possible local metabolism in skin tissues.
Excretion
Triethanolamine salicylate is primarily excreted through the urine after metabolism. Following external application of a 10% formulation, approximately 1.4% of the total dose is recovered in the urine as salicylate within the first 24 hours, indicating low systemic absorption.
- Molecular Formula:C 7H 6O 3.C 6H 15NO 3
- Molecular Wt.: 287.18
INDICATION AND USAGE
Salicor™ is indicated for the temporary relief of minor aches and pains of muscles and joints associated with: arthritis, simple backache, muscle strains and sprains, bruises, bursitis, and dysmenorrhea.
The patch provides a localized analgesic effect directly at the site of pain, offering convenience and sustained relief compared to cream formulations.
When using Salicor™
For External Use Only
Use only as directed.
Do not bandage tightly or use with a heating pad.
Avoid contact with eyes or mucous membranes.
Do not apply to wounds or damaged skin.
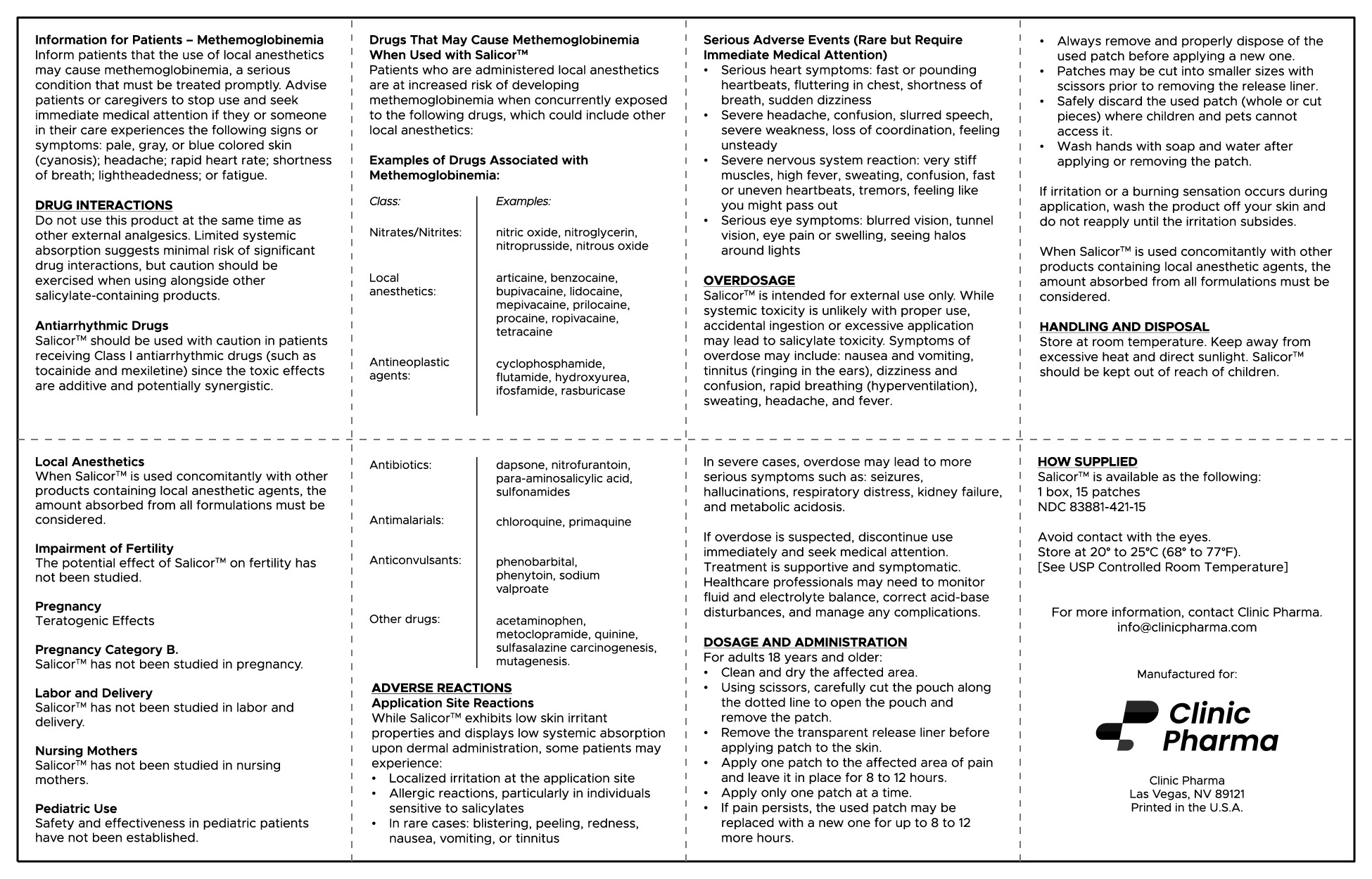
- Do not use this product at the same time as other external analgesics. Limited systemic absorption suggests minimal risk of significant drug interactions, but caution should be exercised when using alongside other salicylate-containing products.
HOW SUPPLIED
Salicor™ is available as the following:
1 box, 15 patches
NDC: 83881-421-15
Avoid contact with the eyes.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature]
For more information, contact Clinic Pharma at info@clinicpharma.com.
Serious Adverse Events (Rare but Require Immediate Medical Attention)
- Severe nervous system reaction: very stiff muscles, high fever, sweating, confusion, fast or uneven heartbeats, tremors, feeling like you might pass out
- Serious eye symptoms: blurred vision, tunnel vision, eye pain or swelling, seeing halos around lights
- Serious heart symptoms: fast or pounding heartbeats, fluttering in chest, shortness of breath, sudden dizziness
- Severe headache, confusion, slurred speech, severe weakness, loss of coordination, feeling unsteady
- In rare cases: blistering, peeling, redness, nausea, vomiting, or tinnitus
INSTRUCTIONS FOR USE:
For adults 18 years and older:
- Clean and dry the affected area.
- Using scissors, carefully cut the pouch along the dotted line to open the pouch and remove the patch.
- Remove the transparent release liner before applying patch to the skin
- Apply one patch to the affected area of pain and leave it in place for 8 to 12 hours.
- Apply only one patch at a time.
- If pain persists, the used patch may be replaced with a new one for up to 8 to 12 more hours.
- Always remove and properly dispose of the used patch before applying a new one.
- Patches may be cut into smaller sizes with scissors prior to removing the release liner.
- Safely discard the used patch (whole or cut pieces) where children and pets cannot access it.
- Wash your hands with soap and water after applying or removing the patch.
- If irritation or a burning sensation occurs during application, wash the product off your skin and do not reapply until the irritation subsides.
- When Salicor™ is used concomitantly with other products containing local anesthetic agents, the amount absorbed from all formulations must be considered.
Visual Guide:

PRECAUTIONS
General
Salicor™ should be used with caution in patients with active gastrointestinal ulceration or bleeding and severe renal or hepatic impairments. Salicor™ should not be applied to open skin wounds, infections, or exfoliative dermatitis.
Stop use and ask a doctor if:
- Condition worsens
- Symptoms persist for more than 7 days, or symptoms return within a few days after discontinuing use
- Redness is present
- Irritation develops
Hepatic Disease
Patients with severe hepatic disease are at greater risk of developing toxic blood concentrations of triethanolamine salicylate because of their inability to metabolize triethanolamine salicylate normally.
Allergic Reactions
Although rare, allergic reactions to oral or external triethanolamine salicylate may occur. Seek emergency medical help if you experience hives, difficulty breathing, or swelling of the face, lips, tongue, or throat, as these may indicate a serious allergic reaction.
Non-intact Skin
Application to broken or inflamed skin, although not tested, may result in higher
blood concentrations of triethanolamine salicylate from increased absorption. Salicor™ is only recommended for use on intact skin.
External Heat Sources
Placement of external heat sources, such as heating pads or electric blankets, over Salicor™ is not recommended, as this has not been evaluated and may increase plasma triethanolamine salicylate levels.
Eye Exposure
Contact of Salicor™ with the eyes, although not studied, should be avoided based on the findings of severe eye irritation with the use of similar products in animals. If eye contact occurs, immediately wash out the eye with water or saline and protect the eye until sensation returns.
HANDLING AND DISPOSAL
Salicor™ should be kept out of reach of children.
- Store at room temperature. Keep away from excessive heat and direct sunlight.
- Safely discard the used patch (whole or cut pieces) where children and pets cannot access it.
- Wash hands with soap and water after applying or removing the patch.
WARNINGS
Medicines intended to be applied to the skin should not be swallowed. Salicor™ is flammable. Keep away from open flame. You should never heat, microwave, or add the medicine to hot water.
CONTRAINDICATIONS
The use of Salicor™ is contraindicated in:
Patients with known hypersensitivity to salicylates or any components of the patch
Patients with an allergy to aspirin
WARNINGS
Medicines intended to be applied to the skin should not be swallowed. Salicor™ is flammable. Keep away from open flame. You should never heat, microwave, or add the medicine to hot water.
Allergy Alert
If prone to allergic reactions from aspirin or salicylates, consult a doctor before use.
For External Use Only
Use only as directed.
Do not bandage tightly or use with a heating pad.
Avoid contact with eyes or mucous membranes.
Do not apply to wounds or damaged skin.
DRUG INTERACTIONS
Do not use this product at the same time as other external analgesics. Limited systemic absorption suggests minimal risk of significant drug interactions, but caution should be exercised when using alongside other salicylate-containing products.
Antiarrhythmic Drugs
Salicor™ should be used with caution in patients receiving Class I antiarrhythmic drugs (such as tocainide and mexiletine) since the toxic effects are additive and potentially synergistic.
Local Anesthetics
When Salicor™ is used concomitantly with other products containing local anesthetic agents, the amount absorbed from all formulations must be considered.
Impairment of Fertility
The potential effect of Salicor™ on fertility has not been studied.
Pregnancy
Teratogenic Effects
Pregnancy Category B.
Salicor™ has not been studied in pregnancy.
Labor and Delivery
Salicor™ has not been studied in labor and delivery.
Nursing Mothers
Salicor™ has not been studied in nursing mothers.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
ADVERSE REACTIONS
Application Site Reactions
While Salicor™ exhibits low skin irritant properties and displays low systemic absorption upon dermal administration, some patients may experience:
Localized irritation at the application site
Allergic reactions, particularly in individuals sensitive to salicylates
In rare cases: blistering, peeling, redness, nausea, vomiting, or tinnitus
Serious Adverse Events (Rare but Require Immediate Medical Attention)
Serious heart symptoms: fast or pounding heartbeats, fluttering in chest, shortness of breath, sudden dizziness
Severe headache, confusion, slurred speech, severe weakness, loss of coordination, feeling unsteady
Severe nervous system reaction: very stiff muscles, high fever, sweating, confusion, fast or uneven heartbeats, tremors, feeling like you might pass out
Serious eye symptoms: blurred vision, tunnel vision, eye pain or swelling, seeing halos around lights
OVERDOSAGE
Salicor™ is intended for external use only. While systemic toxicity is unlikely with proper use, accidental ingestion or excessive application may lead to salicylate toxicity. Symptoms of overdose may include: nausea and vomiting, tinnitus (ringing in the ears), dizziness and confusion, rapid breathing (hyperventilation), sweating, headache, and fever.
In severe cases, overdose may lead to more serious symptoms such as: seizures, hallucinations, respiratory distress, kidney failure, and metabolic acidosis.
If overdose is suspected, discontinue use immediately and seek medical attention. Treatment is supportive and symptomatic. Healthcare professionals may need to monitor fluid and electrolyte balance, correct acid-base disturbances, and manage any complications.
DOSAGE AND ADMINISTRATION
For adults 18 years and older:
Clean and dry the affected area.
Using scissors, carefully cut the pouch along the dotted line to open the pouch and remove the patch.
Remove the transparent release liner before applying patch to the skin.
Apply one patch to the affected area of pain and leave it in place for 8 to 12 hours.
Apply only one patch at a time.
If pain persists, the used patch may be replaced with a new one for up to 8 to 12 more hours. Always remove and properly dispose of the used patch before applying a new one.
Patches may be cut into smaller sizes with scissors prior to removing the release liner.
Safely discard the used patch (whole or cut pieces) where children and pets cannot access it.
Wash your hands with soap and water after applying or removing the patch.
If irritation or a burning sensation occurs during application, wash the product off your skin and do not reapply until the irritation subsides.
When Salicor™ is used concomitantly with other products containing local anesthetic agents, the amount absorbed from all formulations must be considered.
CONTRAINDICATIONS
The use of Salicor™ is contraindicated in:
Patients with known hypersensitivity to salicylates or any components of the patch
Patients with an allergy to aspirin
WARNINGS
Medicines intended to be applied to the skin should not be swallowed. Salicor™ is flammable. Keep away from open flame. You should never heat, microwave, or add the medicine to hot water.
Allergy Alert
If prone to allergic reactions from aspirin or salicylates, consult a doctor before use.
For External Use Only
Use only as directed.
Avoid contact with eyes or mucous membranes.
Do not bandage tightly or use with a heating pad.
Do not apply to wounds or damaged skin.
Risk of Methemoglobinemia
The risk associated with Salicor™ is extremely low to nonexistent. Methemoglobinemia is more commonly linked to the use of local anesthetics like benzocaine, lidocaine, or tetracaine, rather than salicylates such as triethanolamine salicylate. However, while triethanolamine salicylate itself is not typically associated with methemoglobinemia, caution should always be exercised when using any new medication, especially in patients predisposed to this condition, such as those with G6PD deficiency.
PRECAUTIONS
General
Salicor™ should be used with caution in patients with active gastrointestinal ulceration or bleeding and severe renal or hepatic impairments. Salicor™ should not be applied to open skin wounds, infections, or exfoliative dermatitis.
Stop use and ask a doctor if
Condition worsens
Symptoms persist for more than 7 days, or symptoms return within a few days after discontinuing use
Redness is present
Irritation develops
Hepatic Disease
Patients with severe hepatic disease are at greater risk of developing toxic blood concentrations of triethanolamine salicylate because of their inability to metabolize triethanolamine salicylate normally.
Allergic Reactions
Although rare, allergic reactions to oral or external triethanolamine salicylate may occur. Seek emergency medical help if you experience hives, difficulty breathing, or swelling of the face, lips, tongue, or throat, as these may indicate a serious allergic reaction.
Non-intact Skin
Although not tested, application to broken or inflamed skin may result in higher blood concentrations of triethanolamine salicylate from increased absorption. Salicor™ is only recommended for use on intact skin.
External Heat Sources
Placement of external heat sources, such as heating pads or electric blankets, over Salicor™ is not recommended, as this has not been evaluated and may increase plasma triethanolamine salicylate levels.
Eye Exposure
Although not studied, contact of Salicor™ with the eyes should be avoided based on the findings of severe eye irritation with the use of similar animal products. If eye contact occurs, immediately wash out the eye with water or saline and protect the eye until sensation returns.
Information for Patients Methemoglobinemia
Inform patients that the use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to stop use and seek immediate medical attention if they or someone in their care experiences the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Inactive Ingredients
ISOPROPYL ALCOHOL (UNII: ND2M416302)
KOLLIDON SR (UNII: S34RY76LK6)
2-ETHYLHEXYL ACRYLATE-METHYL ACRYLATE-GLYCIDYL METHACRYLATE-ACRYLIC ACID COPOLYMER (FOR DURO-TAK 387-2353) (UNII: 737PT7E2CY)
HEPTANE (UNII: 456148SDMJ)
| SALICOR
triethanolamine salicylate patch |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Clinic Pharma (119158469) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.