ALLERGY RELIEF- chlorpheniramine maleate tablet
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by Chain Drug Consortium, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
-
Directions
adults and children 12
years of age and over1 tablet every 4 to 6 hours. Do
not take more than 6 tablets in 24 hours.children 6 to under 12
years of age1/2 tablet (break tablet in half)
every 4 to 6 hours. Do not
exceed 3 whole tablets in 24 hours.children under 6 years of age do not use this product in
children under 6 years of age
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
Premier
Value®NDC: 68016-683-82
*COMPARE TO THE ACTIVE INGREDIENT IN
CHLOR-TRIMETON® ALLERGY TABLETS4
hour
reliefAllergy Relief
Chlorpheniramine maleate 4 mg
ANTIHISTAMINERelieves:
Sneezing Itchy, Watery Eyes
Runny Nose Itchy Throat24 Tablets
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING*This product is not manufactured or distributed by MSD
Consumer Care, Inc., owner of the registered trademark
Chlor-Trimeton® Allergy Tablets. 50844 ORG021319408DISTRIBUTED BY
CHAIN DRUG CONSORTIUM, LLC
UPARC, BLDG. A3, SUITE 338
1020 WILLIAM PITT WAY
PITTSBURGH, PA 15238
www.chaindrugconsortium.comIf for any reason you are not satisfied with
this product, please return it to the store
where purchased for a full refund.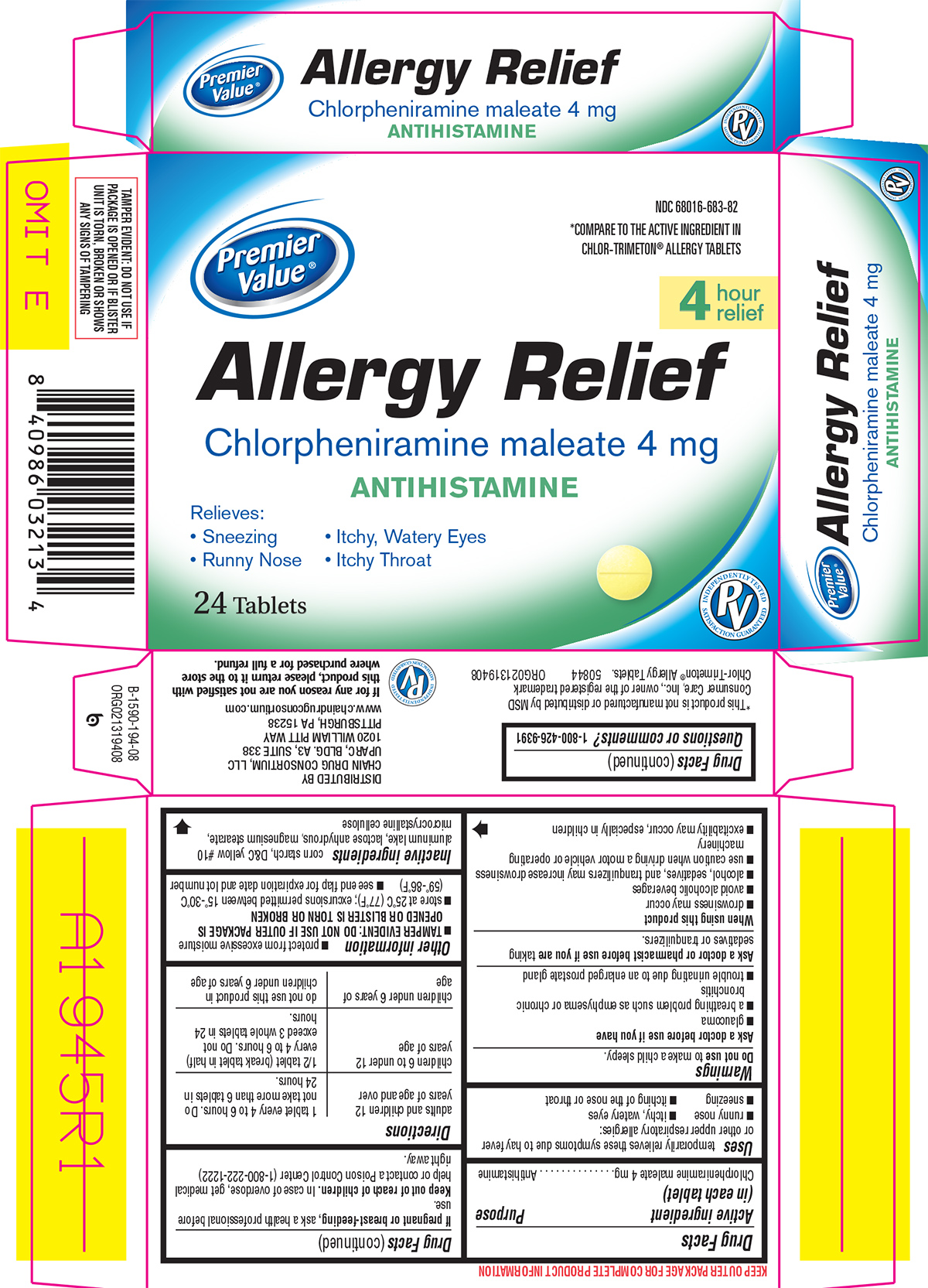
Premier Value 44-194
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
chlorpheniramine maleate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68016-683 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 4 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color YELLOW Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code 44;194 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68016-683-82 2 in 1 CARTON 12/19/1992 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 68016-683-81 1 in 1 CARTON 12/19/1992 2 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 12/19/1992 Labeler - Chain Drug Consortium (101668460) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(68016-683) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(68016-683) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(68016-683) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(68016-683) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(68016-683)
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.