PAIN AND FEVER RELIEF CHILDRENS STRENGTH- acetaminophen tablet, chewable
Pain and Fever Relief by
Drug Labeling and Warnings
Pain and Fever Relief by is a Otc medication manufactured, distributed, or labeled by ScripsAmerica, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
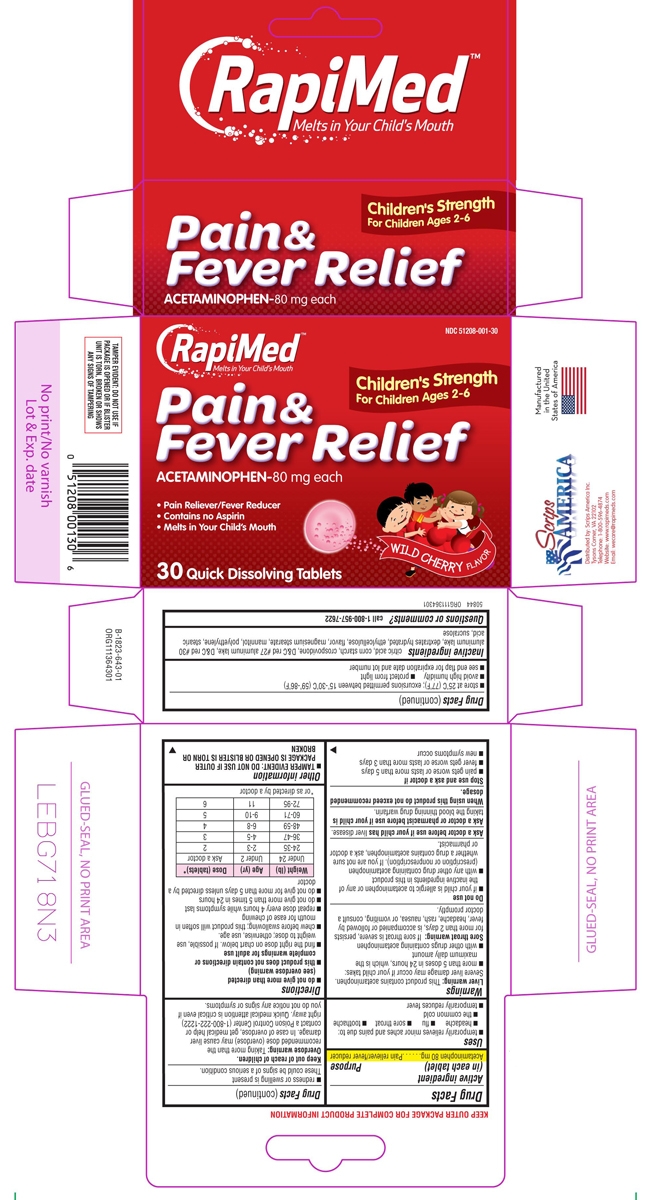
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes:
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Stop use and ask a doctor if
- pain gets worse or lasts more than 5 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical even if you do not notice any signs or symptoms.
-
Directions
- do not give more than directed (see overdose warning)
- this product does not contain directions or complete warnings for adult use
- find the right dose on chart below. If possible, use weight to dose; otherwise, use age.
- chew before swallowing; this product will soften in mouth for ease of chewing
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- do not give for more than 5 days unless directed by a doctor
Weight (lb) Age (yr) Dose (tablets)* Under 24 Under 2 Ask a doctor 24-35 2-3 2 36-47 4-5 3 48-59 6-8 4 60-71 9-10 5 72-95 11 6 *or as directed by a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
RapiMed™
Melts in Your Child's MouthNDC: 51208-001-30
Children's Strength
For Children Ages 2-6Pain &
Fever Relief
ACETAMINOPHEN-80 mg eachPain Reliever/Fever Reducer
Contains no Aspirin
Melts in Your Child's Mouth30 Quick Dissolving Tablets
WILD CHERRY FLAVOR
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERINGManufactured
in the United
States of AmericaDistributed by: Scrips America Inc.
Tysons Corner, VA 22102
Telephone: 1-800-596-4874
Website: www.rapimeds.com
Email: wecare@rapimeds.com50844 ORG111364301
Scrips America 44-643
-
INGREDIENTS AND APPEARANCE
PAIN AND FEVER RELIEF CHILDRENS STRENGTH
acetaminophen tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51208-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 80 mg Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) STARCH, CORN (UNII: O8232NY3SJ) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) D&C RED NO. 30 (UNII: 2S42T2808B) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STEARIC ACID (UNII: 4ELV7Z65AP) SUCRALOSE (UNII: 96K6UQ3ZD4) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) Product Characteristics Color PINK Score no score Shape ROUND Size 13mm Flavor CHERRY (Wild Cherry) Imprint Code 44;643 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51208-001-30 5 in 1 CARTON 03/18/2014 06/02/2020 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part343 03/18/2014 06/02/2020 Labeler - ScripsAmerica (842014177) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(51208-001) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(51208-001) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(51208-001) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(51208-001) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(51208-001)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.