DELZICOL- mesalamine capsule, delayed release
Delzicol by
Drug Labeling and Warnings
Delzicol by is a Prescription medication manufactured, distributed, or labeled by Carilion Materials Management. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DELZICOL® safely and effectively. See full prescribing information for DELZICOL.
DELZICOL® (mesalamine) delayed-release capsules, for oral use
Initial U.S. Approval: 1987RECENT MAJOR CHANGES
Indications and Usage, Treatment of Mildly to Moderately Active Ulcerative Colitis (1.1) 04/2014 Dosage and Administration, Dosage for Treatment of Mildly to Moderately Active Ulcerative Colitis (2.1) 04/2014 Dosage and Administration, Dosage for Treatment of Mildly to Moderately Active Ulcerative Colitis (2.1) 10/2014 Dosage and Administration, Dosage for Maintenance of Remission of Ulcerative Colitis (2.2) 10/2014 Dosage and Administration, Important Administration Instructions (2.3) 04/2014 Dosage and Administration, Testing Prior to DELZICOL Administration (2.4) 04/2014 INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- For the treatment of mildly to moderately active ulcerative colitis (2.1)
ºAdults: 800 mg three times daily with or without food (2.4 grams/day) for 6 weeks
º Pediatric Patients 12 years or older: Total daily dose is weight-based up to a maximum of 2.4 grams/day with or without food (see table below); twice daily dosing for 6 weeks
Weight Group
(kg)Daily Dose
(mg/kg/day)Maximum Daily Dose
(grams/day)17 to < 33
36 to 71
1.2
33 to < 54
37 to 61
2.0
54 to 90
27 to 44
2.4
- For the maintenance of remission of ulcerative colitis in adults, 1.6 g daily, in divided doses (2.2)
- Do not open, crush, break, or chew the capsules. Swallow whole with water (2.3)
- Two DELZICOL 400 mg capsules should not be substituted with one mesalamine delayed-release 800 mg tablet (2.3)
- Assess children for the ability to swallow capsules (2.3)
- Recommend that renal function be evaluated prior to initiation of DELZICOL (2.4, 5.1)
DOSAGE FORMS AND STRENGTHS
Delayed-release capsules: 400 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
-
Renal Impairment (for example, minimal change nephropathy, acute and chronic interstitial nephritis, renal failure): Assess renal function at beginning of treatment and periodically during treatment (5.1)
-
Mesalamine-induced Acute Intolerance Syndrome: Has been reported. Observe patients closely for worsening of these symptoms while on treatment (5.2)
-
Hypersensitivity Reactions: Use caution when treating patients who are hypersensitive to sulfasalazine. Mesalamine-induced cardiac hypersensitivity reactions (myocarditis and pericarditis) have been reported (5.3)
-
Hepatic Failure: Has been reported in patients with pre-existing liver disease. Use caution when treating patients with liver disease (5.4)
-
Prolonged Gastric Retention in Patients with Upper Gastrointestinal Obstruction: May lead to a delay in onset of action (5.5)
ADVERSE REACTIONS
The most common adverse reactions (observed in greater than or equal to 5% of adults in clinical trials) were abdominal pain, eructation, pain, back pain, rash, dyspepsia, rhinitis, flu syndrome, asthenia, flatulence, vomiting, fever, arthralgia, constipation, and gastrointestinal bleeding (6.1)
Adverse reactions in children were similar (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Warner Chilcott at 1-800-521-8813 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatchDRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2016
- For the treatment of mildly to moderately active ulcerative colitis (2.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Treatment of Mildly to Moderately Active Ulcerative Colitis
1.2 Maintenance of Remission of Ulcerative Colitis in Adults
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Treatment of Mildly to Moderately Active Ulcerative Colitis
2.2 Dosage for Maintenance of Remission of Ulcerative Colitis in Adults
2.3 Important Administration Instructions
2.4 Testing Prior to DELZICOL Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
5.2 Mesalamine-Induced Acute Intolerance Syndrome
5.3 Hypersensitivity Reactions
5.4 Hepatic Failure
5.5 Prolonged Gastric Retention in Patients with Upper Gastrointestinal Obstruction
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
7.2 Azathioprine or 6-mercaptopurine
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Treatment of Mildly to Moderately Active Ulcerative Colitis
14.2 Maintenance of Remission of Ulcerative Colitis
HOW SUPPLIED
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Treatment of Mildly to Moderately Active Ulcerative Colitis
DELZICOL® (mesalamine) delayed-release capsules are indicated for the treatment of mildly to moderately active ulcerative colitis in patients 12 years of age and older.
1.2 Maintenance of Remission of Ulcerative Colitis in Adults
DELZICOL® (mesalamine) delayed-release tablets are indicated for the maintenance of remission of ulcerative colitis in adults. The safety and effectiveness of DELZICOL for the maintenance of remission of ulcerative colitis in pediatric patients have not been established.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Treatment of Mildly to Moderately Active Ulcerative Colitis
Adults
For adults, the recommended dosage of DELZICOL is two 400 mg capsules to be taken three times daily (total daily dose of 2.4 grams), for a duration of 6 weeks [see Clinical Studies (14.1)].Pediatrics
For pediatric patients 12 years of age and older, the recommended total daily dose of DELZICOL is weight-based (up to maximum of 2.4 grams/day). DELZICOL capsules are to be taken twice daily with or without food for a duration of 6 weeks [see Clinical Studies (14.1)]. Pediatric Dosage by Weight Weight Group
(kg)Daily Dose
(mg/kg/day)Maximum Daily Dose
(grams/day)17 to < 33 36 to 71 1.2 33 to < 54 37 to 61 2.0 54 to 90 27 to 44 2.4 2.2 Dosage for Maintenance of Remission of Ulcerative Colitis in Adults
For the maintenance of remission of ulcerative colitis, the recommended dose of DELZICOL in adults is 1.6 g daily with or without food in divided doses [see Clinical Studies (14.2)].
2.3 Important Administration Instructions
Do not open, crush, break, or chew the capsules. Swallow whole with water.
Before prescribing DELZICOL capsules, children should be assessed for the ability to swallow capsules.
Two DELZICOL 400 mg capsules should not be substituted with one mesalamine delayed-release 800 mg tablet.
2.4 Testing Prior to DELZICOL Administration
Evaluate renal function prior to initiation of DELZICOL [see Warnings and Precautions (5.1)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
Renal impairment, including minimal change nephropathy, acute and chronic interstitial nephritis, and renal failure, has been reported in patients taking products such as DELZICOL that contain mesalamine or are converted to mesalamine.
It is recommended that patients have an evaluation of renal function prior to initiation of DELZICOL and periodically while on therapy.
Prescribers should carefully evaluate the risks and benefits when using DELZICOL in patients with known renal impairment or history of renal disease [see Drug Interactions (7.1) and Nonclinical Toxicology (13.2)].
5.2 Mesalamine-Induced Acute Intolerance Syndrome
Mesalamine has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. Although the exact frequency of occurrence has not been determined, it has occurred in 3% of controlled clinical trials of mesalamine or sulfasalazine. Symptoms include cramping, abdominal pain, bloody diarrhea, and sometimes fever, headache, and rash. Observe patients closely for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with DELZICOL.
5.3 Hypersensitivity Reactions
Some patients who have experienced a hypersensitivity reaction to sulfasalazine may have a similar reaction to DELZICOL or to other compounds that contain or are converted to mesalamine.
Mesalamine-induced cardiac hypersensitivity reactions (myocarditis and pericarditis) have been reported with DELZICOL and other mesalamine medications. Caution should be taken in prescribing this medicine to patients with conditions predisposing them to the development of myocarditis or pericarditis.
-
6 ADVERSE REACTIONS
The most serious adverse reactions seen in DELZICOL clinical trials or with other products that contain or are metabolized to mesalamine are:
- Renal impairment, including renal failure [see Warnings and Precautions (5.1)]
- Acute intolerance syndrome [see Warnings and Precautions (5.2)]
- Hypersensitivity reactions [see Warnings and Precautions (5.3)]
- Hepatic failure [see Warnings and Precautions (5.4)]
The data presented in Section 6.1 are from clinical trials conducted with mesalamine delayed-release tablets. DELZICOL is bioequivalent to these mesalamine delayed-release tablets.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In total, mesalamine delayed-release 400 mg tablets have been evaluated in 2690 patients with ulcerative colitis in controlled and open-label trials. Adverse events presented in the following sections may occur regardless of length of therapy and similar events have been reported in short- and long-term studies and in the postmarketing setting.
Clinical studies supporting mesalamine delayed-release tablets use for the treatment of mildly to moderately active ulcerative colitis included two 6-week, placebo-controlled, randomized, double-blind studies in adults with mildly to moderately active ulcerative colitis (Studies 1 and 2), and one 6-week, randomized, double-blind, study of 2 dose levels in children with mildly to moderately active ulcerative colitis. Clinical studies supporting the use of mesalamine delayed-release tablets in the maintenance of remission of ulcerative colitis included a 6-month, randomized, double-blind, placebo-controlled, multi-center study and four active-controlled maintenance trials comparing mesalamine delayed-release with sulfasalazine. Mesalamine delayed-release tablets have been evaluated in 427 adults and 82 children with ulcerative colitis in these controlled studies.
Treatment of Mildly to Moderately Active Ulcerative Colitis in Adults
In two 6-week placebo-controlled clinical studies (Studies 1 and 2) involving 245 patients, 155 of whom were randomized to mesalamine delayed-release tablets [see Clinical Studies (14.1)], 3.2% of the mesalamine delayed-release tablets-treated patients discontinued therapy because of adverse reactions as compared to 2.2% of the placebo-treated patients. The average age of patients in Study 1 was 42 years and 48% of patients were male. The average age of patients in Study 2 was 42 years and 59% of patients were male. Adverse reactions leading to withdrawal from mesalamine delayed-release tablets included (each in one patient): diarrhea and colitis flare; dizziness, nausea, joint pain, and headache; rash, lethargy and constipation; dry mouth, malaise, lower back discomfort, mild disorientation, mild indigestion and cramping; headache, nausea, aching, vomiting, muscle cramps, a stuffy head, plugged ears, and fever.
Adverse reactions in patients treated with mesalamine delayed-release tablets occurring at a frequency of at least 2% and at a rate greater than placebo in 6-week, double-blind, placebo-controlled trials (Studies 1 and 2) are listed in Table 1 below.
Table 1: Adverse Reactions Reported in Two Six-Week Placebo-Controlled Trials (Studies 1 and 2) Experienced by at Least 2% of patients in the mesalamine delayed-release tablets Group and at a Rate Greater than Placebo % of Patients with Adverse Reactions Adverse Reaction mesalamine delayed-release tablets Placebo (n = 152) (n = 87) Abdominal pain 18 14 Eructation 16 15 Pain 14 8 Back pain 7 5 Rash 6 3 Dyspepsia 6 1 Arthralgia 5 3 Vomiting 5 2 Constipation 5 1 Chest pain 3 2 Chills 3 2 Peripheral edema 3 2 Myalgia 3 1 Sweating 3 1 Pruritus 3 0 Acne 2 1 Malaise 2 1 Arthritis 2 0 Treatment of Mildly to Moderately Active Ulcerative Colitis in Pediatric Patients 5 to 17 Years Old
A randomized, double-blind, 6-week study of 2 dose levels of mesalamine delayed-release 400 mg tablets (Study 3) was conducted in 82 pediatric patients 5 to 17 years of age with mildly to moderately active ulcerative colitis. All patients were divided by body weight category (17 to less than 33 kg, 33 to less than 54 kg, and 54 to 90 kg) and randomly assigned to receive a low dose (1.2, 2.0, and 2.4 g/day for the respective body weight category) or a high dose (2.0, 3.6, and 4.8 g/day).
The high dose is not an approved dosage because it was not found to be more effective than the approved dose [see Dosage and Administration (2.1) and Clinical Studies (14.1)].
Duration of exposure to mesalamine among the 82 patients in the study ranged from 12 to 50 days (mean of 40 days in each dose group). The majority (88%) of patients in each group were treated for more than 5 weeks. Table 2 provides a summary of the specific reported adverse reactions (ARs).
Table 2: Adverse Reactions Reported in One Six-Week Trial (Study 3) Experienced by at Least 5% of Patients in the Low Dose Group or High Dose Group % of Patients with Adverse Reactions Low Dose High Dose Adverse Reaction (n=41) (n=41) Nasopharyngitis 15 12 Ulcerative Colitis 12 5 Headache 10 5 Abdominal pain 10 2 Dizziness 7 2 Sinusitis 7 0 Rash 5 5 Cough 5 0 Diarrhea 5 0 Fatigue 2 10 Pyrexia 0 7 Increased Lipase 0 5
Low Dose = mesalamine 400 mg delayed-release tablet 1.2 - 2.4 g/day;
High Dose = mesalamine 400 mg delayed-release tablet 2.0 - 4.8 g/day.
Dosage was dependent on body weight.
Adverse Reactions reported at the 1-week telephone follow-up visit are included.Twelve percent of the patients in the low dose group and 5% of the patients in the high dose group had serious adverse reactions (ARs). Ulcerative colitis was reported as a serious AR in one subject in each group. Other serious ARs consisted of sinusitis, abdominal pain, decreased body mass index, adenovirus infection, bloody diarrhea, sclerosing cholangitis, and pancreatitis in one subject each in the low dose group and anemia and syncope in one subject each in the high dose group.
Seven patients were withdrawn from the study because of ARs: 5 (12%) in the low dose group (ulcerative colitis, adenovirus infection, sclerosing cholangitis, pancreatitis) and 2 (5%) in the high dose group (increased amylase and increased lipase, upper abdominal pain).
In general, the nature and severity of reactions in the pediatric population was similar to those reported in adult populations of patients with ulcerative colitis.
Maintenance of Remission of Ulcerative Colitis in AdultsIn a 6-month placebo-controlled maintenance trial involving 264 patients (Study 4) 177 of whom were randomized to mesalamine delayed-release tablets, six (3.4%) of the patients using mesalamine delayed-release tablets discontinued therapy because of adverse reactions, as compared to four (4.6%) of patients using placebo [see Clinical Studies (14.2)]. The average age of patients in Study 4 was 42 years and 55% of patients were male. Adverse reactions leading to study withdrawal in patients using mesalamine delayed-release tablets included (each in one patient): anxiety; headache; pruritus; decreased libido; rheumatoid arthritis; and stomatitis and asthenia.
In addition to reactions listed in Table 1, the following adverse reactions occurred in patients using mesalamine delayed-release tablets at a frequency of 2% or greater in Study 4: abdominal enlargement, gastroenteritis, gastrointestinal hemorrhage, infection, joint disorder, migraine, nervousness, paresthesia, rectal disorder, rectal hemorrhage, stool abnormalities, tenesmus, urinary frequency, vasodilation, and vision abnormalities.
In 3342 patients in uncontrolled clinical studies, the following adverse reactions occurred at a frequency of 5% or greater and appeared to increase in frequency with increasing dose: asthenia, fever, flu syndrome, pain, abdominal pain, back pain, flatulence, gastrointestinal bleeding, arthralgia, and rhinitis.
6.2 Postmarketing Experience
In addition to the adverse reactions reported above in clinical trials involving mesalamine delayed-release tablets, the adverse reactions listed below have been identified during post-approval use of mesalamine delayed-release tablets and other mesalamine-containing products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: Neck pain, facial edema, edema, lupus-like syndrome, drug fever.
Cardiovascular: Pericarditis, myocarditis [see Warnings and Precautions (5.3)].
Gastrointestinal: Anorexia, pancreatitis, gastritis, increased appetite, cholecystitis, dry mouth, oral ulcers, perforated peptic ulcer bloody diarrhea.
Hematologic: Agranulocytosis, aplastic anemia, thrombocytopenia, eosinophilia, leukopenia, anemia, lymphadenopathy.
Musculoskeletal: Gout.
Nervous: Depression, somnolence, emotional lability, hyperesthesia, vertigo, confusion, tremor, peripheral neuropathy, transverse myelitis, Guillain-Barré syndrome.
Renal: Renal failure, interstitial nephritis, minimal change nephropathy [see Warnings and Precautions (5.1)].
Respiratory/Pulmonary: Eosinophilic pneumonia, interstitial pneumonitis, asthma exacerbation, pleuritis.
Skin: Alopecia, psoriasis, pyoderma gangrenosus, dry skin, erythema nodosum, urticaria.
Special Senses: Eye pain, taste perversion, blurred vision, tinnitus.
Urogenital: Dysuria, urinary urgency, hematuria, epididymitis, menorrhagia, reversible oligospermia.
Laboratory Abnormalities: Elevated AST (SGOT) or ALT (SGPT), elevated alkaline phosphatase, elevated GGT, elevated LDH, elevated bilirubin, elevated serum creatinine and BUN.
-
7 DRUG INTERACTIONS
No formal drug interaction studies have been performed using DELZICOL with other drugs. However, the following interactions between mesalamine-containing products and other drugs have been reported.
7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
The concurrent use of mesalamine with known nephrotoxic agents, including nonsteroidal anti-inflammatory drugs (NSAIDs) may increase the risk of renal reactions [see Warnings and Precautions (5.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Risk Summary
There are no adequate and well controlled studies of DELZICOL use in pregnant women. Limited published human data on mesalamine show no increase in the overall rate of congenital malformations. Some data show an increased rate of preterm birth, stillbirth, and low birth weight; however, these adverse pregnancy outcomes are also associated with active inflammatory bowel disease. Furthermore, all pregnancies, regardless of drug exposure, have a background rate of 2 to 4% for major malformations, and 15 to 20% for pregnancy loss. No evidence of fetal harm was observed in animal reproduction studies of mesalamine in rats and rabbits at oral doses approximately 1.9 times (rat) and 3.9 times (rabbit) the recommended human dose. DELZICOL should be used in pregnancy only if clearly needed.Human Data
Mesalamine crosses the placenta. In prospective and retrospective studies of over 600 women exposed to mesalamine during pregnancy, the observed rate of congenital malformations was not increased above the background rate in the general population. Some data show an increased rate of preterm birth, stillbirth, and low birth weight, but it is unclear whether this was due to underlying maternal disease, drug exposure, or both, as active inflammatory bowel disease is also associated with adverse pregnancy outcomes.Animal data
Reproduction studies with mesalamine were performed during organogenesis in rats and rabbits at oral doses up to 480 mg/kg/day. There was no evidence of impaired fertility or harm to the fetus. These mesalamine doses were about 1.9 times (rat) and 3.9 times (rabbit) the recommended human dose, based on body surface area.8.3 Nursing Mothers
Mesalamine and its N-acetyl metabolite are present in human milk. In published lactation studies, maternal mesalamine doses from various oral and rectal formulations and products ranged from 500 mg to 3 g daily. The concentration of mesalamine in milk ranged from non-detectable to 0.11 mg/L. The concentration of the N-acetyl-5-aminosalicylic acid metabolite ranged from 5 to 18.1 mg/L. Based on these concentrations, estimated infant daily doses for an exclusively breastfed infant are 0 to 0.017 mg/kg/day of mesalamine and 0.75 to 2.72 mg/kg/day of N-acetyl-5-aminosalicylic acid. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for DELZICOL and any potential adverse effects on the breastfed child from the drug or from the underlying maternal condition. Caution should be exercised when DELZICOL is administered to a nursing woman.
8.4 Pediatric Use
The data presented in Section 8.4 are from clinical trials conducted with mesalamine 400 mg delayed-release tablets. DELZICOL is bioequivalent to these mesalamine delayed-release tablets.
The safety and effectiveness of mesalamine delayed-release 400 mg tablets in pediatric patients 5 to 17 years of age for treatment of mildly to moderately active ulcerative colitis have been established over a 6-week period. Use of mesalamine delayed-release 400 mg tablets in these age groups is supported by evidence from adequate and well controlled studies of mesalamine delayed-release 400 mg tablets in adults and a single study in pediatric patients [see Adverse Reactions (6.1), Clinical Pharmacology (12.3) and Clinical Studies (14.1)]. However, for patients less than 12 years of age, there is no age-appropriate formulation available. Therefore, DELZICOL is indicated for the treatment of mildly to moderately active ulcerative colitis for patients 12 years of age and older.
Mesalamine delayed-release 400 mg tablets was studied in a randomized, double-blind, parallel-group, 6-week treatment study of two dose levels of mesalamine delayed-release 400 mg tablets in 82 pediatric patients 5 to 17 years of age with mildly to moderately active ulcerative colitis. All patients were divided by weight category (17 to less than 33 kg, 33 to less than 54 kg, and 54 to 90 kg) and randomly assigned to receive a low dose (1.2, 2.0, and 2.4 g/day for the respective weight category) or a high dose (2.0, 3.6, and 4.8 g/day). Baseline and screening visits were followed by a treatment period of 6 weeks [see Dosage and Administration (2.1)]. The high dose was not more effective than the low dose and is not an approved dosage [see Clinical Studies (14.1)].
The safety and effectiveness of DELZICOL in pediatric patients below the age of 5 years have not been established. The safety and effectiveness of DELZICOL in the maintenance of remission of ulcerative colitis in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of mesalamine delayed-release tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger patients. In general, the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients should be considered when prescribing DELZICOL. Reports from uncontrolled clinical studies and postmarketing reporting systems suggest a higher incidence of blood dyscrasias, that is, agranulocytosis, neutropenia, pancytopenia, in subjects receiving mesalamine delayed-release tablets who are 65 years or older. Caution should be taken to closely monitor blood cell counts during treatment with DELZICOL.
8.6 Renal Impairment
Mesalamine is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken when prescribing this drug therapy. It is recommended that all patients have an evaluation of renal function prior to initiation of DELZICOL therapy and periodically while on DELZICOL therapy [see Dosage and Administration (2.4) and Warnings and Precautions (5.1)].
-
10 OVERDOSAGE
There is no specific antidote for mesalamine overdose and treatment for suspected acute severe toxicity with DELZICOL should be symptomatic and supportive. This may include prevention of further gastrointestinal tract absorption, correction of fluid electrolyte imbalance, and maintenance of adequate renal function. DELZICOL is a pH dependent delayed-release product and this factor should be considered when treating a suspected overdose.
-
11 DESCRIPTION
Each DELZICOL (mesalamine) delayed-release capsule for oral administration contains 400 mg of mesalamine, an aminosalicylate. DELZICOL (mesalamine) delayed-release capsules contain acrylic based resin, Eudragit S (methacrylic acid copolymer type B, NF), which dissolves at pH 7 or greater and releases mesalamine in the terminal ileum and beyond for topical anti-inflammatory action in the colon. Mesalamine (also referred to as 5-aminosalicylic acid or 5-ASA) has the chemical name 5-amino-2-hydroxybenzoic acid. Its structural formula is:
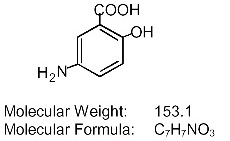
Mesalamine structural formula
Inactive Ingredients: Each capsule contains colloidal silicon dioxide, dibutyl sebacate, ferric oxide red, ferric oxide yellow, lactose monohydrate, magnesium stearate, methacrylic acid copolymer type B (Eudragit S), polyethylene glycol, povidone, sodium starch glycolate, talc and hydroxypropyl methylcellulose (HPMC).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of mesalamine is unknown, but appears to be topical rather than systemic. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase pathways, that is, prostanoids, and through the lipoxygenase pathways, that is, leukotrienes and hydroxyeicosatetraenoic acids, is increased in patients with chronic ulcerative colitis, and it is possible that mesalamine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin production in the colon.
12.3 Pharmacokinetics
Absorption
Approximately 28% of mesalamine in mesalamine delayed-release formulations is absorbed after oral ingestion. The Tmax for mesalamine and its metabolite, is usually delayed, reflecting the delayed-release, and ranges from 4 to 16 hours.A high fat meal increased systemic exposure of mesalimine (geometric mean Cmax: ↑ 64%; AUC: ↑ 48%) and delayed the Tmax by 3.3 hours compared to results in the fasted state. A majority (85 - 90%) of subjects in the fed state had systemic exposures within the range noted in the fasted state, with the exception of a few that had much higher exposures due to unknown reasons. The observed differences in mesalamine exposure due to concomitant food intake are not considered to be clinically relevant at the total daily dose of 2.4 g/day. Therefore DELZICOL can be taken without regard to food.
Metabolism
The absorbed mesalamine is rapidly acetylated in the gut mucosal wall and by the liver to N-acetyl-5-aminosalicylic acid.Excretion
Absorbed mesalamine is excreted mainly by the kidney as N-acetyl-5-aminosalicylic acid. Unabsorbed mesalamine is excreted in feces.After intravenous administration, the elimination half-life of mesalamine is reported to be approximately 40 minutes. After oral dosing, the terminal t1/2 values for mesalamine and N-acetyl-5-aminosalicylic acid are usually about 12 hours, but are variable, ranging from 2 to 15 hours. There is a large inter-subject and intra-subject variability in the plasma concentrations of mesalamine and N-acetyl-5-aminosalicylic acid and in their terminal half-lives following administration of DELZICOL.
Specific Populations
Pediatric Patients
The pediatric data presented in Section 12 are from clinical trials conducted with mesalamine delayed-release 400 mg tablets. DELZICOL is bioequivalent to these mesalamine delayed-release tablets.In a PK study evaluating 30, 60 and 90 mg/kg/day doses of mesalamine delayed-release 400 mg tablets administered twice daily for four weeks, the mean Cavg values of mesalamine in pediatric ulcerative colitis patients ranged from approximately 400 ng/mL to 2100 ng/mL based on data from all dose levels.
In a study in pediatric ulcerative colitis patients (Study 3), mean plasma concentrations of mesalamine (based on sparse sampling) were 820 to 988 ng/mL at the low dose level (that is, 1.2, 2.0 or 2.4 g/day based on body weight strata of 17 to less than 33 kg, 33 to less than 54 kg, and 54 to 90 kg, respectively).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Mesalamine was not carcinogenic at dietary doses of up to 480 mg/kg/day in rats and 2000 mg/kg/day in mice, which are about 2.9 and 6.1 times the maximum recommended maintenance dose of DELZICOL of 1.6 g/day or 26.7 mg/kg/day, based on 60 kg body weight, respectively, based on body surface area.Mutagenesis
Mesalamine was negative in the Ames assay for mutagenesis, negative for induction of sister chromatid exchanges (SCE) and chromosomal aberrations in Chinese hamster ovary cells in vitro, and negative for induction of micronuclei (MN) in mouse bone marrow polychromatic erythrocytes.Impairment of Fertility
Mesalamine, at oral doses up to 480 mg/kg/day (about 1.9 times the recommended human treatment dose on a body surface area basis), was found to have no effect on fertility or reproductive performance of male and female rats.13.2 Animal Toxicology and/or Pharmacology
In animal studies (rats, mice, dogs), the kidney was the principal organ for toxicity. (In the following, comparisons of animal dosing to recommended human dosing are based on body surface area and a 2.4 g/day dose for a 60 kg person.)
Mesalamine causes renal papillary necrosis in rats at single doses of approximately 750 mg/kg to 1000 mg/kg (approximately 3 to 4 times the recommended human dose based on body surface area). Doses of 170 and 360 mg/kg/day (about 0.7 and 1.5 times the recommended human dose based on body surface area) given to rats for six months produced papillary necrosis, papillary edema, tubular degeneration, tubular mineralization, and urothelial hyperplasia.
In mice, oral doses of 4000 mg/kg/day mesalamine (approximately 8 times the recommended human dose based on body surface area) for three months produced tubular nephrosis, multifocal/diffuse tubulo-interstitial inflammation, and multifocal/diffuse papillary necrosis.
In dogs, single doses of 6000 mg (approximately 8 times the recommended human dose based on body surface area) of delayed-release mesalamine tablets resulted in renal papillary necrosis but were not fatal. Renal changes have occurred in dogs given chronic administration of mesalamine at doses of 80 mg/kg/day (1.1 times the recommended human dose based on body surface area).
-
14 CLINICAL STUDIES
The data presented in Section 14 are from clinical trials conducted with mesalamine delayed-release tablets. DELZICOL is bioequivalent to these mesalamine delayed-release tablets.
14.1 Treatment of Mildly to Moderately Active Ulcerative Colitis
Two placebo-controlled studies (Studies 1 and 2) have demonstrated the efficacy of mesalamine delayed-release tablets in patients with mildly to moderately active ulcerative colitis.
In one randomized, double-blind, multi-center trial of 158 patients (Study 1), mesalamine delayed-release doses of 1.6 g/day and 2.4 g/day for 6 weeks were compared to placebo. The scoring system for determination of treatment efficacy included assessment of stool frequency, rectal bleeding, sigmoidoscopic findings, patient’s functional assessment, and physician global assessment. At the dose of 2.4 g/day, 21 of 43 (49%) patients using mesalamine delayed-release tablets showed an improvement in sigmoidoscopic appearance of the bowel compared to 12 of 44 (27%) patients using placebo (p = 0.048). In addition, significantly more patients in mesalamine delayed-release tablets 2.4 g/day group showed improvement in rectal bleeding and stool frequency. The 1.6 g/day dose did not produce consistent evidence of effectiveness.
In a second randomized, double-blind, placebo-controlled clinical trial of 6 weeks duration in 87 (Study 2) patients, mesalamine delayed-release tablets, at a dose of 4.8 g/day, for 6 weeks, resulted in sigmoidoscopic improvement in 28 of 38 (74%) patients compared to 10 of 38 (26%) placebo patients (p less than 0.001). Also, more patients in the mesalamine delayed-release tablets 4.8 g/day group than the placebo group showed improvement in overall symptoms.
The 4.8 g/day dose is not an approved dosage for the treatment of mildly to moderately active ulcerative colitis.
Pediatrics
The safety and effectiveness of mesalamine delayed-release 400 mg tablets in pediatric patients 5 to 17 years of age for treatment of mildly to moderately active ulcerative colitis are supported by evidence from adequate and well controlled studies of mesalamine delayed-release 400 mg tablets in adults and a single study in pediatric patients.A randomized, double-blind, 6-week study of 2 dose levels of mesalamine delayed-release 400 mg tablets (Study 3) was conducted in 82 pediatric patients 5 to 17 years of age with mildly or moderately active ulcerative colitis. All patients were divided by weight category (17 to less than 33 kg, 33 to less than 54 kg, and 54 to 90 kg) and randomly assigned to receive a low dose (1.2, 2.0, and 2.4 g/day for the respective weight category) or a high dose (2.0, 3.6, and 4.8 g/day). Doses were administered every 12 hours.
The proportion of patients who achieved success based on the Truncated Mayo Score (TM-Mayo) (based on the stool frequency and rectal bleeding subscores of the Mayo Score) and based on the Pediatric Ulcerative Colitis Activity Index (PUCAI) (which included assessment of abdominal pain, rectal bleeding, stool consistency and frequency, presence of nocturnal bowel movement and level of activity) was measured after 6 weeks of treatment. Success based on TM-Mayo was defined as either partial response (improvement from baseline in stool frequency or rectal bleeding subscores with no worsening in the other) or complete response (both stool frequency and rectal bleeding subscores equal 0). Success based on PUCAI was defined as either partial response (PUCAI reduction of greater than or equal to 20 points from Baseline to Week 6 with Week 6 score greater than or equal to 10) or complete response (PUCAI less than 10 at Week 6).
There were 41 patients in the low dose group and 41 patients in the high dose group who received at least one dose of mesalamine delayed-release 400 mg tablets; 36 patients in each dose group completed the study. Patients were considered treatment failures if they did not achieve success or dropped out due to adverse reaction or lack of efficacy.
At Week 6, 73.2% of the patients in the low dose group, and 70.0% of the patients in the high dose group achieved success based on the TM-Mayo; 34.1% of the patients in the low dose group and 42.5% of the patients in the high dose group achieved complete response. At Week 6, 56.1% of the patients in the low dose group, and 55.0% of the patients in the high dose group achieved success based on the PUCAI; 46.3% of the patients in the low dose group and 42.5% of the patients in the high dose group achieved complete response.
The high dose was not more effective than the low dose and is not an approved dosage [see Dosage and Administration (2.1)].
14.2 Maintenance of Remission of Ulcerative Colitis
A 6-month, randomized, double-blind, placebo-controlled, multi-center study (Study 4) involved 264 patients treated with mesalamine delayed-release tablets 0.8 g/day (n = 90), 1.6 g/day (n = 87), or placebo (n = 87). In the 0.8 g/day arm, patients were dosed twice daily; in the 1.6 g/day arm, patients were dosed four times daily. The proportion of patients treated with 0.8 g/day who maintained endoscopic remission was not statistically significant compared to placebo. The proportion of patients using mesalamine delayed-release tablets 1.6 g/day who maintained endoscopic remission of ulcerative colitis was in 61 of 87 (70.1%) compared with 42 of 87 (48.3%) of placebo patients (p = 0.005).
A pooled efficacy analysis of 4 maintenance trials compared mesalamine delayed-release tablets, at doses of 0.8 g/day to 2.8 g/day, in divided doses ranging from twice daily to four times per day, with sulfasalazine, at doses of 2 g/day to 4 g/day. Treatment success was seen in 59 of 98 (59%) patients using mesalamine delayed-release tablets and 70 of 102 (69%) of patients using sulfasalazine, a non-significant difference.
- HOW SUPPLIED
-
17 PATIENT COUNSELING INFORMATION
- Instruct patients to swallow the DELZICOL capsule whole with water, taking care not to open, break, crush, or chew the capsules, because the coating is an important part of the delayed-release formulation.
- Instruct patients to contact their physician if they are unable to swallow the DELZICOL capsules whole.
- Inform patients that if they are switching from a previous oral mesalamine therapy to DELZICOL they should discontinue their previous oral mesalamine therapy and follow the dosing instructions for DELZICOL. Inform patients that intact, partially intact, and/or capsule shells have been reported in the stool. Instruct patients to contact their physician if this occurs repeatedly.
- Instruct patients to protect DELZICOL from moisture. Instruct patients to close the container tightly and to leave any desiccant pouches present in the bottle along with the capsules.
Manufactured By:
Warner Chilcott Deutschland GmbH
D-64331 Weiterstadt
GermanyMarketed By:
Warner Chilcott (US), LLC
Rockaway, NJ 07866
Content Updated: October 2014
10001367 - Mesalamine 400 mg DR caps
-
INGREDIENTS AND APPEARANCE
DELZICOL
mesalamine capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68151-5652(NDC: 0430-0753) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MESALAMINE (UNII: 4Q81I59GXC) (MESALAMINE - UNII:4Q81I59GXC) MESALAMINE 400 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIBUTYL SEBACATE (UNII: 4W5IH7FLNY) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:2) (UNII: 5KY68S2577) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POVIDONES (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) HYPROMELLOSES (UNII: 3NXW29V3WO) Product Characteristics Color RED Score no score Shape CAPSULE Size 22mm Flavor Imprint Code WC;400mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68151-5652-2 1 in 1 PACKAGE; Type 0: Not a Combination Product 03/01/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204412 03/01/2013 Labeler - Carilion Materials Management (079239644) Establishment Name Address ID/FEI Business Operations Carilion Materials Management 079239644 REPACK(68151-5652)
Trademark Results [Delzicol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DELZICOL 85808925 4372504 Live/Registered |
ALLERGAN PHARMACEUTICALS INTERNATIONAL LIMITED 2012-12-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
