IPOL (poliovirus type 1 antigen (formaldehyde inactivated), poliovirus type 2 antigen (formaldehyde inactivated), and poliovirus type 3 antigen- formaldehyde inactivated injection, suspension
IPOL by
Drug Labeling and Warnings
IPOL by is a Other medication manufactured, distributed, or labeled by Sanofi Pasteur Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
IPOL®, Poliovirus Vaccine Inactivated, produced by Sanofi Pasteur SA, is a sterile suspension of three types of poliovirus: Type 1 (Mahoney), Type 2 (MEF-1), and Type 3 (Saukett). IPOL vaccine is a highly purified, inactivated poliovirus vaccine with enhanced potency. Each of the three strains of poliovirus is individually grown in vero cells, a continuous line of monkey kidney cells cultivated on microcarriers. (1) (2) The cells are grown in Eagle MEM modified medium, supplemented with newborn calf bovine serum tested for adventitious agents prior to use, originated from countries free of bovine spongiform encephalopathy. For viral growth, the culture medium is replaced by M-199, without calf bovine serum. This culture technique and improvements in purification, concentration, and standardization of poliovirus antigen produce a more potent and consistent immunogenic vaccine than the inactivated poliovirus vaccine (IPV) available in the US prior to 1988. (3) (4)
After clarification and filtration, viral suspensions are concentrated by ultrafiltration, and purified by three liquid chromatography steps; one column of anion exchanger, one column of gel filtration, and again one column of anion exchanger. After re-equilibration of the purified viral suspension with Medium M-199 and adjustment of the antigen titer, the monovalent viral suspensions are inactivated at +37°C for at least 12 days with 1:4000 formalin.
Each dose (0.5 mL) of trivalent vaccine is formulated to contain 40 D antigen units of Type 1, 8 D antigen units of Type 2, and 32 D antigen units of Type 3 poliovirus. For each lot of IPOL vaccine, D-antigen content is determined in vitro using the D-antigen ELISA assay. IPOL vaccine is produced from vaccine concentrates diluted with M-199 medium. Also present are 0.5% of 2-phenoxyethanol and a maximum of 0.02% of formaldehyde per dose as preservatives. Neomycin, streptomycin, and polymyxin B are used in vaccine production; and, although purification procedures eliminate measurable amounts, less than 5 ng neomycin, 200 ng streptomycin, and 25 ng polymyxin B per dose may still be present. The residual calf bovine serum albumin is less than 50 ng/dose in the final vaccine.
The vaccine is clear and colorless and should be administered intramuscularly or subcutaneously.
The vial stopper is not made with natural rubber latex.
-
CLINICAL PHARMACOLOGY
Poliomyelitis is caused by poliovirus Types 1, 2, or 3. It is primarily spread by the fecal-oral route of transmission but may also be spread by the pharyngeal route.
Approximately 90% to 95% of poliovirus infections are asymptomatic. Nonspecific illness with low-grade fever and sore throat (minor illness) occurs in 4% to 8% of infections. Aseptic meningitis occurs in 1% to 5% of patients a few days after the minor illness has resolved. Rapid onset of asymmetric acute flaccid paralysis occurs in 0.1% to 2% of infections, and residual paralytic disease involving motor neurons (paralytic poliomyelitis) occurs in approximately 1 per 1,000 infections. (5)
Prior to the introduction of inactivated poliovirus vaccines in 1955, large outbreaks of poliomyelitis occurred each year in the United States (US). The annual incidence of paralytic disease of 11.4 cases/100,000 population declined to 0.5 cases by the time oral poliovirus vaccine (OPV) was introduced in 1961. Incidence continued to decline thereafter to a rate of 0.002 to 0.005 cases per 100,000 population. Of the 127 cases of paralytic poliomyelitis reported in the US between 1980 and 1994, six were imported cases (caused by wild polioviruses), two were "indeterminate" cases, and 119 were vaccine associated paralytic poliomyelitis (VAPP) cases associated with the use of live, attenuated oral poliovirus vaccine (OPV). (6) An all IPV schedule was adopted in 1999 to eliminate VAPP cases. (7)
Poliovirus Vaccine Inactivated induces the production of neutralizing antibodies against each type of virus which are related to protective efficacy. Antibody response in most children was induced after receiving fewer doses (8) of IPV vaccine than the vaccine available in the United States prior to 1988.
Studies in developed (8) and developing (9), (10) countries with a similar enhanced IPV manufactured by the same process as IPOL vaccine in primary monkey kidney cells have shown a direct relationship exists between the antigenic content of the vaccine, the frequency of seroconversion, and resulting antibody titer. Approval in the US was based upon demonstration of immunogenicity and safety in US children. (11)
In the US, 219 infants received three doses of a similar enhanced IPV at two, four, and eighteen months of age manufactured by the same process as IPOL vaccine except the cell substrate for IPV was using primary monkey kidney cells. Seroconversion to all three types of poliovirus was demonstrated in 99% of these infants after two doses of vaccine given at 2 and 4 months of age. Following the third dose of vaccine at 18 months of age, neutralizing antibodies were present at a level of ≥1:10 in 99.1% of children to Type 1 and 100% of children to Types 2 and 3 polioviruses. (3)
IPOL vaccine was administered to more than 700 infants between 2 to 18 months of age during three clinical studies conducted in the US using IPV only schedules and sequential IPV-OPV schedules. (12) (13) Seroprevalence rates for detectable serum neutralizing antibody (DA) at a ≥1:4 dilution were 95% to 100% (Type 1); 97% to 100% (Type 2) and 96% to 100% (Type 3) after two doses of IPOL vaccine depending on studies.
Table 1: US Studies with IPOL Vaccine Administered Using IPV Only or Sequential IPV-OPV Schedules Age (months) for Post Dose 2 Post Dose 3 Pre Booster Post Booster 2 4 6 12 to 18 Type 1 Type 2 Type 3 Type 1 Type 2 Type 3 Type 1 Type 2 Type 3 Type 1 Type 2 Type 3 Dose 1 Dose 2 Dose 3 Booster N* %DA† %DA %DA N* %DA %DA %DA N* %DA %DA %DA N* %DA %DA %DA I IPOL vaccine given either separately in association with DTP in two sites (s) or combined (c) with DTP in a dual chambered syringe O OPV - * N = Number of children from whom serum was available
- † Detectable antibody (neutralizing titer ≥1:4)
- ‡ IPOL vaccine given subcutaneously
- § NA – No poliovirus vaccine administered
- ¶ IPOL vaccine given intramuscularly
STUDY 1 (11)‡ I(s) I(s) NA§ I(s) 56 97 100 97 – – – 53 91 97 93 53 97 100 100 O O NA O 22 100 100 100 – – – 22 78 91 78 20 100 100 100 I(s) O NA O 17 95 100 95 – – – 17 95 100 95 17 100 100 100 I(s) I(s) NA O 17 100 100 100 – – – 16 100 100 94 16 100 100 100 STUDY 2 (10)¶ I(c) I(c) NA I(s) 94 98 97 96 – – – 100 92 95 88 97 100 100 100 I(s) I(s) NA I(s) 68 99 100 99 – – – 72 100 100 94 75 100 100 100 I(c) I(c) NA O 75 95 99 96 – – – 77 86 97 82 78 100 100 97 I(s) I(s) NA O 101 99 99 95 – – – 103 99 97 89 107 100 100 100 STUDY 3 (10)¶ I(c) I(c) I(c) O 91 98 99 100 91 100 100 100 41 100 100 100 40 100 100 100 I(c) I(c) O O 96 100 98 99 94 100 100 99 47 100 100 100 45 100 100 100 I(c) I(c) I(c) + O O 91 96 97 100 85 100 100 100 47 100 100 100 46 100 100 100 In one study, (13) the persistence of DA in infants receiving two doses of IPOL vaccine at 2 and 4 months of age was 91% to 100% (Type 1), 97% to 100% (Type 2), and 93% to 94% (Type 3) at twelve months of age. In another study, (12) 86% to 100% (Type 1), 95% to 100% (Type 2), and 82% to 94% (Type 3) of infants still had DA at 18 months of age.
In trials and field studies conducted outside the US, IPOL vaccine, or a combination vaccine containing IPOL vaccine and DTP, was administered to more than 3,000 infants between 2 to 18 months of age using IPV only schedules and immunogenicity data are available from 1,485 infants. After two doses of vaccine given during the first year of life, seroprevalence rates for detectable serum neutralizing antibody (neutralizing titer ≥1:4) were 88% to 100% (Type 1); 84% to 100% (Type 2) and 94% to 100% (Type 3) of infants, depending on studies. When three doses were given during the first year of life, post-dose 3 DA ranged between 93% to 100% (Type 1); 89% to 100% (Type 2) and 97% to 100% (Type 3) and reached 100% for Types 1, 2, and 3 after the fourth dose given during the second year of life (12 to 18 months of age). (14)
In infants immunized with three doses of an unlicensed combination vaccine containing IPOL vaccine and DTP given during the first year of life, and a fourth dose given during the second year of life, the persistence of detectable neutralizing antibodies was 96%, 96%, and 97% against poliovirus Types 1, 2, and 3, respectively, at six years of age. DA reached 100% for all types after a booster dose of IPOL vaccine combined with DTP vaccine. (11) A survey of Swedish children and young adults given a Swedish IPV only schedule demonstrated persistence of detectable serum neutralizing antibody for at least 10 years to all three types of poliovirus. (15)
IPV is able to induce secretory antibody (IgA) produced in the pharynx and gut and reduces pharyngeal excretion of poliovirus Type 1 from 75% in children with neutralizing antibodies at levels less than 1:8 to 25% in children with neutralizing antibodies at levels more than 1:64. (4) (14) (16) (17) (18) (19) (20) (21) (22) There is also evidence of induction of herd immunity with IPV, (15) (23) (24) (25) (26) and that this herd immunity is sufficiently maintained in a population vaccinated only with IPV. (26)
VAPP has not been reported in association with administration of IPOL vaccine. (27) It is expected that an IPV only schedule will eliminate the risk of VAPP in both recipients and contacts compared to a schedule that included OPV. (7)
-
INDICATIONS AND USAGE
IPOL vaccine is indicated for active immunization of infants (as young as 6 weeks of age), children, and adults for the prevention of poliomyelitis caused by poliovirus Types 1, 2, and 3. (28)
INFANTS, CHILDREN AND ADOLESCENTS
General Recommendations
It is recommended that all infants (as young as 6 weeks of age), unimmunized children, and adolescents not previously immunized be vaccinated routinely against paralytic poliomyelitis. (29) Following the eradication of poliomyelitis caused by wild poliovirus from the Western Hemisphere (including North and South America) (30), an IPV-only schedule was recommended to eliminate VAPP. (7)
All children should receive four doses of IPV at ages 2, 4, 6 to 18 months, and 4 to 6 years. OPV is no longer available in the US and is not recommended for routine immunization. (7)
Previous clinical poliomyelitis (usually due to only a single poliovirus type) or incomplete immunization with OPV are not contraindications to completing the primary series of immunization with IPOL vaccine.
Children Incompletely Immunized
Children of all ages should have their immunization status reviewed and be considered for supplemental immunization as follows for adults. Time intervals between doses longer than those recommended for routine primary immunization do not necessitate additional doses as long as a final total of four doses is reached (see DOSAGE AND ADMINISTRATION section).
ADULTS
General Recommendations
Routine primary poliovirus vaccination of adults (generally those 18 years of age or older) residing in the US is not recommended. Unimmunized adults who are potentially exposed to wild poliovirus and have not been adequately immunized should receive polio vaccination in accordance with the schedule given in the DOSAGE AND ADMINISTRATION section. (28)
Persons with previous wild poliovirus disease who are incompletely immunized or unimmunized should be given additional doses of IPOL vaccine if they fall into one or more categories listed.
The following categories of adults are at an increased risk of exposure to wild polioviruses: (28) (31)
- Travelers to regions or countries where poliomyelitis is endemic or epidemic.
- Healthcare workers in close contact with patients who may be excreting polioviruses.
- Laboratory workers handling specimens that may contain polioviruses.
- Members of communities or specific population groups with disease caused by wild polioviruses.
IMMUNODEFICIENCY AND ALTERED IMMUNE STATUS
IPOL vaccine should be used in all patients with immunodeficiency diseases and members of such patients' households when vaccination of such persons is indicated. This includes patients with asymptomatic HIV infection, AIDS or AIDS-Related Complex, severe combined immunodeficiency, hypogammaglobulinemia, or agammaglobulinemia; altered immune states due to diseases such as leukemia, lymphoma, or generalized malignancy; or an immune system compromised by treatment with corticosteroids, alkylating drugs, antimetabolites or radiation. Immunogenicity of IPOL vaccine in individuals receiving immunoglobulin could be impaired, and patients with an altered immune state may or may not develop a protective response against paralytic poliomyelitis after administration of IPV. (32)
As with any vaccine, vaccination with IPOL vaccine may not protect 100% of individuals.
Use with other vaccines: refer to DOSAGE AND ADMINISTRATION section for this information.
-
CONTRAINDICATIONS
IPOL vaccine is contraindicated in persons with a history of hypersensitivity to any component of the vaccine, including 2-phenoxyethanol, formaldehyde, neomycin, streptomycin, and polymyxin B.
No further doses should be given if anaphylaxis or anaphylactic shock occurs within 24 hours of administration of one dose of vaccine.
Vaccination of persons with an acute, febrile illness should be deferred until after recovery; however, minor illness, such as mild upper respiratory infection, with or without low grade fever, are not reasons for postponing vaccine administration.
-
WARNINGS
Neomycin, streptomycin, polymyxin B, 2-phenoxyethanol, and formaldehyde are used in the production of this vaccine. Although purification procedures eliminate measurable amounts of these substances, traces may be present (see DESCRIPTION section), and allergic reactions may occur in persons sensitive to these substances (see CONTRAINDICATIONS section).
Systemic adverse reactions reported in infants receiving IPV concomitantly at separate sites or combined with DTP have been similar to those associated with administration of DTP alone. (11) Local reactions are usually mild and transient in nature.
Although no causal relationship between IPOL vaccine and Guillain-Barré Syndrome (GBS) has been established, (28) GBS has been temporally related to administration of another inactivated poliovirus vaccine. Deaths have been reported in temporal association with the administration of IPV (see ADVERSE REACTIONS section).
-
PRECAUTIONS
GENERAL
Prior to an injection of any vaccine, all known precautions should be taken to prevent adverse reactions. This includes a review of the patient's history with respect to possible sensitivity to the vaccine or similar vaccines.
Healthcare providers should question the patient, parent or guardian about reactions to a previous dose of this product, or similar product.
Epinephrine injection (1:1000) and other appropriate agents should be available to control immediate allergic reactions.
Healthcare providers should obtain the previous immunization history of the vaccinee, and inquire about the current health status of the vaccinee.
Immunodeficient patients or patients under immunosuppressive therapy may not develop a protective immune response against paralytic poliomyelitis after administration of IPV.
Administration of IPOL vaccine is not contraindicated in individuals infected with HIV. (33) (34) (35)
Special care should be taken to ensure that the injection does not enter a blood vessel.
INFORMATION FOR PATIENTS
Patients, parents, or guardians should be instructed to report any serious adverse reactions to their healthcare provider.
The healthcare provider should inform the patient, parent, or guardian of the benefits and risks of the vaccine.
The healthcare provider should inform the patient, parent, or guardian of the importance of completing the immunization series.
The healthcare provider should provide the Vaccine Information Statements (VISs) which are required to be given with each immunization.
DRUG INTERACTIONS
There are no known interactions of IPOL vaccine with drugs or foods. Concomitant administration of other parenteral vaccines, with separate syringes at separate sites, is not contraindicated. The first two doses of IPOL vaccine may be administered at separate sites using separate syringes concomitantly with DTaP, acellular pertussis, Haemophilus influenzae type b (Hib), and hepatitis B vaccines. From historical data on the antibody responses to diphtheria, tetanus, acellular pertussis, Hib, or hepatitis B vaccines used concomitantly or in combination with IPOL vaccine, no interferences have been observed on the immunological end points accepted for clinical protection. (11) (16) (36) (See DOSAGE AND ADMINISTRATION section.)
If IPOL vaccine has been administered to persons receiving immunosuppressive therapy, an adequate immunologic response may not be obtained. (See PRECAUTIONS – GENERAL section.)
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
Long-term studies in animals to evaluate carcinogenic potential or impairment of fertility have not been conducted.
PREGNANCY
Animal reproduction studies have not been conducted with IPOL vaccine. It is also not known whether IPOL vaccine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. IPOL vaccine should be given to a pregnant woman only if clearly needed.
NURSING MOTHERS
It is not known whether IPOL vaccine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when IPOL vaccine is administered to a nursing woman.
PEDIATRIC USE
SAFETY AND EFFECTIVENESS OF IPOL VACCINE IN INFANTS BELOW SIX WEEKS OF AGE HAVE NOT BEEN ESTABLISHED. (12) (20) (See DOSAGE AND ADMINISTRATION section.)
In the US, infants receiving two doses of IPV at 2 and 4 months of age, the seroprevalence to all three types of poliovirus was demonstrated in 95% to 100% of these infants after two doses of vaccine. (12) (13)
-
ADVERSE REACTIONS
Body System As A Whole
In earlier studies with the vaccine grown in primary monkey kidney cells, transient local reactions at the site of injection were observed. (3) Erythema, induration and pain occurred in 3.2%, 1% and 13%, respectively, of vaccinees within 48 hours post-vaccination. Temperatures of ≥39°C (≥102°F) were reported in 38% of vaccinees. Other symptoms included irritability, sleepiness, fussiness, and crying. Because IPV was given in a different site but concurrently with Diphtheria and Tetanus Toxoids and Pertussis Vaccine Adsorbed (DTP), these systemic reactions could not be attributed to a specific vaccine. However, these systemic reactions were comparable in frequency and severity to that reported for DTP given alone without IPV. (12) Although no causal relationship has been established, deaths have occurred in temporal association after vaccination of infants with IPV. (37)
Four additional US studies using IPOL vaccine in more than 1,300 infants, (12) between 2 to 18 months of age administered with DTP at the same time at separate sites or combined have demonstrated that local and systemic reactions were similar when DTP was given alone.
Table 2 (12): Percentage of Infants Presenting with Local or Systemic Reactions at 6, 24, and 48 Hours of Immunization with IPOL Vaccine Administered Intramuscularly Concomitantly at Separate Sites with Sanofi* Whole-Cell DTP Vaccine at 2 and 4 Months of Age and with Sanofi Acellular Pertussis Vaccine (Tripedia®) at 18 Months of Age AGE AT IMMUNIZATION REACTION 2 Months
(n=211)4 Months
(n=206)18 Months†
(n=74)6 Hrs. 24 Hrs. 48 Hrs. 6 Hrs. 24 Hrs. 48 Hrs. 6 Hrs. 24 Hrs. 48 Hrs. - * Sanofi Pasteur Inc. formerly known as Aventis Pasteur Inc.
- † Children who have been vaccinated with Tripedia vaccine.
- ‡ Data are from the IPOL vaccine administration site, given intramuscularly.
- § The adverse reaction profile includes the concomitant use of Sanofi whole-cell DTP vaccine or Tripedia vaccine with IPOL vaccine. Rates are comparable in frequency and severity to that reported for whole-cell DTP given alone.
Local, IPOL vaccine alone‡ Erythema >1" 0.5% 0.5% 0.5% 1.0% 0.0% 0.0% 1.4% 0.0% 0.0% Swelling 11.4% 5.7% 0.9% 11.2% 4.9% 1.9% 2.7% 0.0% 0.0% Tenderness 29.4% 8.5% 2.8% 22.8% 4.4% 1.0% 13.5% 4.1% 0.0% Systemic§ Fever >102.2°F 1.0% 0.5% 0.5% 2.0% 0.5% 0.0% 0.0% 0.0% 4.2% Irritability 64.5% 24.6% 17.5% 49.5% 25.7% 11.7% 14.7% 6.7% 8.0% Tiredness 60.7% 31.8% 7.1% 38.8% 18.4% 6.3% 9.3% 5.3% 4.0% Anorexia 16.6% 8.1% 4.3% 6.3% 4.4% 2.4% 2.7% 1.3% 2.7% Vomiting 1.9% 2.8% 2.8% 1.9% 1.5% 1.0% 1.3% 1.3% 0.0% Persistent Crying Percentage of infants within 72 hours after immunization was 0.0% after dose one, 1.4% after dose two, and 0.0% after dose three. Post-marketing Experience
The following adverse events have been identified during postapproval use of IPOL vaccine. Because these events are reported voluntarily from a population of uncertain size, it may not be possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure. Adverse events were included based on one or more of the following factors: severity, frequency of reporting or strength of evidence for a causal relationship.
- Blood and lymphatic system disorders: lymphadenopathy
- General disorders and administration site conditions: agitation, injection site reaction including injection site rash and mass
- Immune system disorders: type I hypersensitivity including allergic reaction, anaphylactic reaction, and anaphylactic shock
- Musculoskeletal and connective tissue disorders: arthralgia, myalgia
- Nervous system disorders: convulsion, febrile convulsion, headache, paresthesia, and somnolence
- Skin and subcutaneous tissue disorders: rash, urticaria
Reporting of Adverse Events
The National Vaccine Injury Compensation Program, established by the National Childhood Vaccine Injury Act of 1986, requires physicians and other healthcare providers who administer vaccines to maintain permanent vaccination records and to report occurrences of certain adverse events to the US Department of Health and Human Services. Reportable events include those listed in the Act for each vaccine and events specified in the package insert as contraindications to further doses of that vaccine. (38) (39) (40)
Reporting by parents or guardians of all adverse events after vaccine administration should be encouraged. Adverse events following immunization with vaccine should be reported by healthcare providers to the US Department of Health and Human Services (DHHS) Vaccine Adverse Event Reporting System (VAERS). Reporting forms and information about reporting requirements or completion of the form can be obtained from VAERS through a toll-free number 1-800-822-7967. (38) (39) (40)
Healthcare providers also should report these events to the Pharmacovigilance Department, Sanofi Pasteur Inc., Discovery Drive, Swiftwater, PA 18370 or call 1-800-822-2463.
-
DOSAGE AND ADMINISTRATION
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The vial and its packaging should be inspected prior to use for evidence of leakage or a faulty seal. If evidence of such defects are observed, the vaccine should not be used. Do not remove the vial stopper or the metal seal holding it in place.
After preparation of the injection site, using a suitable sterile needle and aseptic technique, immediately administer IPOL vaccine intramuscularly or subcutaneously. In infants and small children, the mid-lateral aspect of the thigh is the preferred site. In older children and adults, IPOL vaccine should be administered intramuscularly or subcutaneously in the deltoid area. IPOL should not be combined through reconstitution or mixed with any other vaccine.
To help avoid HIV (AIDS), HBV (Hepatitis), and other infectious diseases due to accidental needlesticks, contaminated needles should not be recapped or removed, unless there is no alternative or that such action is required by a specific medical procedure.
Care should be taken to avoid administering the injection into or near blood vessels and nerves. If blood or any suspicious discoloration appears in the syringe, do not inject but discard contents and repeat procedures using a new dose of vaccine administered at a different site.
DO NOT ADMINISTER VACCINE INTRAVENOUSLY.
Children
The primary series of IPOL vaccine consists of three 0.5 mL doses administered intramuscularly or subcutaneously, preferably eight or more weeks apart and usually at ages 2, 4, and 6 to 18 months. Under no circumstances should the vaccine be given more frequently than four weeks apart. The first immunization may be administered as early as six weeks of age. For this series, a booster dose of IPOL vaccine is administered at 4 to 6 years of age. (41)
Use with Other Vaccines
From historical data on the antibody responses to diphtheria, tetanus, whole-cell or acellular pertussis, Hib, or hepatitis B vaccines used concomitantly with IPOL vaccine, no interferences have been observed on the immunological end points accepted for clinical protection. (11) (16) (36) (See DRUG INTERACTIONS section.)
If the third dose of IPOL vaccine is given between 12 to 18 months of age, it may be desirable to administer this dose with Measles, Mumps, and Rubella (MMR) vaccine and/or other vaccines using separate syringes at separate sites, (28) but no data on the immunological interference between IPOL vaccine and these vaccines exist.
Use in Previously Vaccinated Children
Children and adolescents with a previously incomplete series of polio vaccine should receive sufficient additional doses of IPOL vaccine to complete the series.
Interruption of the recommended schedule with a delay between doses does not interfere with the final immunity. There is no need to start the series over again, regardless of the time elapsed between doses.
The need to routinely administer additional doses is unknown at this time. (28)
Adults
Unvaccinated Adults
A primary series of IPOL vaccine is recommended for unvaccinated adults at increased risk of exposure to poliovirus. While the responses of adults to primary series have not been studied, the recommended schedule for adults is two 0.5 mL doses given at a 1 to 2 month interval and a third 0.5 mL dose given 6 to 12 months later. If less than 3 months but more than 2 months are available before protection is needed, three doses of IPOL vaccine should be given at least 1 month apart. Likewise, if only 1 or 2 months are available, two 0.5 mL doses of IPOL vaccine should be given at least 1 month apart. If less than 1 month is available, a single 0.5 mL dose of IPOL vaccine is recommended. (28)
Incompletely Vaccinated Adults
Adults who are at an increased risk of exposure to poliovirus and who have had at least one dose of OPV, fewer than three doses of conventional IPV or a combination of conventional IPV or OPV totaling fewer than three doses should receive at least one 0.5 mL dose of IPOL vaccine. Additional doses needed to complete a primary series should be given if time permits. (28)
Completely Vaccinated Adults
Adults who are at an increased risk of exposure to poliovirus and who have previously completed a primary series with one or a combination of polio vaccines can be given a 0.5 mL dose of IPOL vaccine.
The preferred injection site of IPOL vaccine for adults is in the deltoid area.
-
HOW SUPPLIED
Vial containing ten 0.5 mL doses: NDC: 49281-860-78. Supplied as package: NDC: 49281-860-10.
-
REFERENCES
- 1 van Wezel AL, et al. Inactivated poliovirus vaccine: Current production methods and new developments. Rev Infect Dis 6 (Suppl 2): S335-S340, 1984.
- 2 Montagnon BJ, et al. Industrial scale production of inactivated poliovirus vaccine prepared by culture of Vero cells on microcarrier. Rev Infect Dis 6 (Suppl 2): S341-S344, 1984.
- 3 McBean AM, et al. Serologic response to oral polio vaccine and enhanced-potency inactivated polio vaccines. Am J Epidemiol 128: 615-628, 1988.
- 4 Murdin AD, et al. Inactivated poliovirus vaccine: past and present experience. Vaccine 8: 735-746, 1996.
- 5 Sabin AB. Poliomyelitis. In Brande AI, Davis CE, Fierer J (eds) International Textbook of Medicine, Vol II. Infectious Diseases and Medical Microbiology. 2nd ed. Philadelphia, WB Saunders, 1986.
- 6 Prevots DR, et al. Vaccine-associated paralytic poliomyelitis in the United States, l980-1994: current risk and potential impact of a proposed sequential schedule of IPV followed by OPV (Abstract #H90). In: Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, DC. American Society for Microbiology, 179, 1996.
- 7 ACIP. Updated Recommendations of the Advisory Committee on Immunization Practices. Poliomyelitis Prevention in the United States. MMWR 49: No. RR-5, 2000.
- 8 Salk J, et al. Antigen content of inactivated poliovirus vaccine for use in a one- or two-dose regimen. Ann Clin Res 14: 204-212, 1982.
- 9 Salk J, et al. Killed poliovirus antigen titration in humans. Develop Biol Standard 41: 119-132, 1978.
- 10 Salk J, et al. Theoretical and practical considerations in the application of killed poliovirus vaccine for the control of paralytic poliomyelitis. Develop Biol Standard 47: 181-198, 1981.
- 11 Unpublished data available from Sanofi Pasteur SA.
- 12 Unpublished data available from Sanofi Pasteur Inc.
- 13 Faden H, et al. Comparative evaluation of immunization with live attenuated and enhanced potency inactivated trivalent poliovirus vaccines in childhood: Systemic and local immune responses. J Infect Dis 162: 1291-1297, 1990.
- 14 Vidor E, et al. The place of DTP/eIPV vaccine in routine pædiatric vaccination. Rev Med Virol 4: 261-277, 1994.
- 15 Bottiger M. Long-term immunity following vaccination with killed poliovirus vaccine in Sweden, a country with no circulating poliovirus. Rev Infect Dis 6 (Suppl 2): S548-S551, 1984.
- 16 Plotkin SA, et al. Inactivated polio vaccine for the United States: a missed vaccination opportunity. Pediatr Infect Dis J 14: 835-839, 1995.
- 17 Marine WM, et al. Limitation of fecal and pharyngeal poliovirus excretion in Salk-vaccinated children. A family study during a Type 1 poliomyelitis epidemic. Amer J Hyg 76: 173-195, 1962.
- 18 Bottiger M, et al. Vaccination with attenuated Type 1 poliovirus, the Chat strain. II. Transmission of virus in relation to age. Acta Paed Scand 55: 416-421, 1966.
- 19 Dick GWA, et al. Vaccination against poliomyelitis with live virus vaccines. Effect of previous Salk vaccination on virus excretion. Brit Med J 2: 266-269, 1961.
- 20 Wehrle PF, et al. Transmission of poliovirus; III. Prevalence of polioviruses in pharyngeal secretions of infected household contacts of patients with clinical disease. Pediatrics 27: 762-764, 1961.
- 21 Adenyi-Jones SC, et al. Systemic and local immune responses to enhanced-potency inactivated poliovirus vaccine in premature and term infants. J Pediatr 120: No 5, 686-689, 1992.
- 22 Chin TDY. Immunity induced by inactivated poliovirus vaccine and excretion of virus. Rev Infect Dis 6 (Suppl 2): S369-S370, 1984.
- 23 Salk D. Herd effect and virus eradication with use of killed poliovirus vaccine. Develop Biol Standard 47: 247-255, 1981.
- 24 Bijerk H. Surveillance and control of poliomyelitis in the Netherlands. Rev Infect Dis 6 (Suppl 2): S451-S456, 1984.
- 25 Lapinleimu K. Elimination of poliomyelitis in Finland. Rev Infect Dis 6 (Suppl 2): S457-S460, 1984.
- 26 Conyn van Spaendonck M, et al. Circulation of Poliovirus during the poliomyelitis outbreak in the Netherlands in 1992-1993. Amer J Epidemiology 143: 929-935, 1996.
- 27 Strebel PM, et al. Epidemiology of poliomyelitis in the United States one decade after the last reported case of indigenous wild virus associated disease. Clin Infect Dis 14: 568-579, 1992.
- 28 ACIP. Poliomyelitis prevention in the United States: introduction of a sequential vaccination schedule of Inactivated Poliovirus Vaccine followed by Oral Poliovirus Vaccine. MMWR 46: No. RR-3, 1997.
- 29 WHO. Weekly Epidemiology Record 54: 82-83, 1979.
- 30 Certification of poliomyelitis eradication - the Americas, 1994. MMWR 43: 720-722, 1994.
- 31 Institute of Medicine. An evaluation of poliomyelitis vaccine poliomyelitis vaccine policy options. Washington, DC. National Academy of Sciences, 1988.
- 32 ACIP. Immunization of children infected with human T-lymphotropic virus type III/lymphadenopathy-associated virus. MMWR 35: 595-606, 1986.
- 33 ACIP. General recommendations on immunization. MMWR 43: No. RR-1, 1994.
- 34 Barbi M, et al. Antibody response to inactivated polio vaccine (eIPV) in children born to HIV positive mothers. Eur J Epidemiol 8: 211-216, 1992.
- 35 Varon D, et al. Response to hemophilic patients to poliovirus vaccination: Correlation with HIV serology and with immunological parameters. J Med Virol 40: 91-95, 1993.
- 36 Vidor E, et al. Fifteen-years experience with vero-produced enhanced potency inactivated poliovirus vaccine (eIPV). Ped Infect Dis J, 312-322, 1997.
- 37 Stratton, R. et al. Adverse Events Associated with Childhood Vaccines. Polio Vaccines. National Academy Press, 295-299, 1994.
- 38 CDC. Vaccine Adverse Event Reporting System - United States. MMWR 39: 730-733, 1990.
- 39 CDC. National Childhood Vaccine Injury Act. Requirements for permanent vaccination records and for reporting of selected events after vaccination. MMWR 37: 197-200, 1988.
- 40 Food & Drug Administration. New Reporting Requirements for Vaccine Adverse Events. FDA Drug Bull 18 (2), 16-18, 1988.
- 41 Recommended childhood immunization schedule - United States, 1999. MMWR 48: 12-16, 1999.
- SPL UNCLASSIFIED SECTION
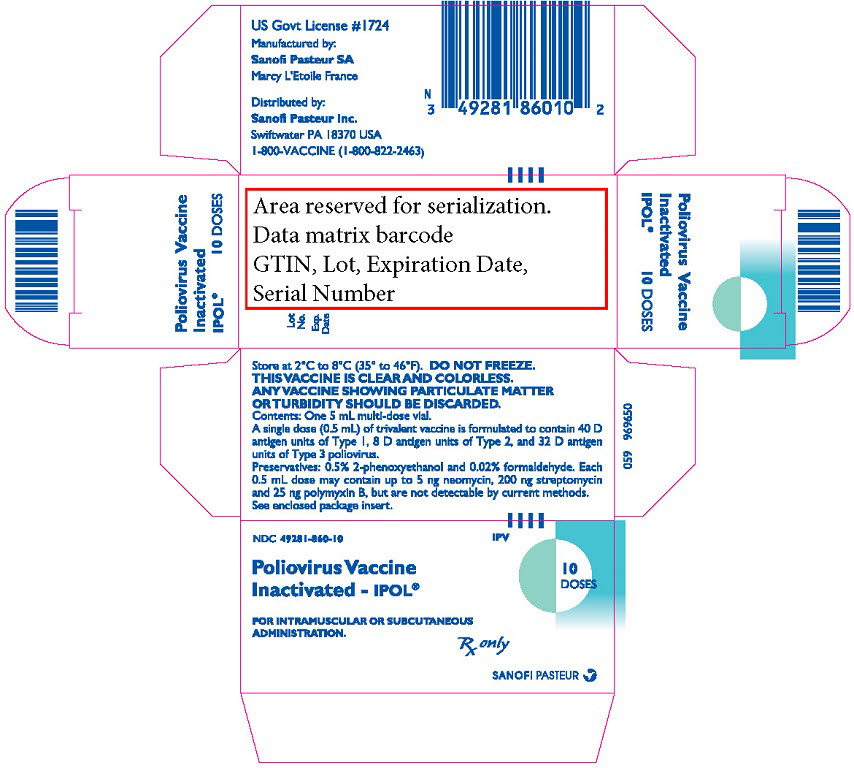
- PRINCIPAL DISPLAY PANEL - 10 Dose Vial Label
-
PRINCIPAL DISPLAY PANEL - 10 Dose Vial Package
NDC: 49281-860-10
IPVPoliovirus Vaccine
Inactivated - IPOL®FOR INTRAMUSCULAR OR SUBCUTANEOUS
ADMINISTRATION.10
DOSESRx only
SANOFI PASTEUR
-
INGREDIENTS AND APPEARANCE
IPOL
poliovirus type 1 antigen (formaldehyde inactivated), poliovirus type 2 antigen (formaldehyde inactivated), and poliovirus type 3 antigen (formaldehyde inactivated) injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC: 49281-860 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLIOVIRUS TYPE 1 ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: 0LVY784C09) (POLIOVIRUS TYPE 1 ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:0LVY784C09) POLIOVIRUS TYPE 1 ANTIGEN (FORMALDEHYDE INACTIVATED) 40 [D'ag'U] in 0.5 mL POLIOVIRUS TYPE 2 ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: 23JE9KDF4R) (POLIOVIRUS TYPE 2 ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:23JE9KDF4R) POLIOVIRUS TYPE 2 ANTIGEN (FORMALDEHYDE INACTIVATED) 8 [D'ag'U] in 0.5 mL POLIOVIRUS TYPE 3 ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: 459ROM8M9M) (POLIOVIRUS TYPE 3 ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:459ROM8M9M) POLIOVIRUS TYPE 3 ANTIGEN (FORMALDEHYDE INACTIVATED) 32 [D'ag'U] in 0.5 mL Inactive Ingredients Ingredient Name Strength PHENOXYETHANOL (UNII: HIE492ZZ3T) 3 uL in 0.5 mL FORMALDEHYDE (UNII: 1HG84L3525) 20 ug in 0.5 mL NEOMYCIN (UNII: I16QD7X297) 5 ng in 0.5 mL STREPTOMYCIN (UNII: Y45QSO73OB) 200 ng in 0.5 mL POLYMYXIN B (UNII: J2VZ07J96K) 25 ng in 0.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49281-860-10 1 in 1 PACKAGE 1 NDC: 49281-860-78 5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103930 12/21/1990 Labeler - Sanofi Pasteur Inc. (086723285) Establishment Name Address ID/FEI Business Operations Sanofi Pasteur Inc. 578763542 MANUFACTURE
Trademark Results [IPOL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IPOL 90262574 not registered Live/Pending |
Fang Baojun 2020-10-19 |
 IPOL 85391553 not registered Dead/Abandoned |
Powervation Inc. 2011-08-06 |
 IPOL 75571559 not registered Dead/Abandoned |
Pluris, Inc. 1998-10-16 |
 IPOL 74110857 1745040 Live/Registered |
SANOFI PASTEUR LIMITED/SANOFI PASTEUR LIMITEE 1990-10-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
