COLUMVI- glofitamab concentrate COLUMVI- glofitamab solution, concentrate
Columvi by
Drug Labeling and Warnings
Columvi by is a Prescription medication manufactured, distributed, or labeled by Genentech, Inc., Roche Diagnostics, Genentech, Inc. (Oceanside), Roche Diagnostics GmbH, F. Hoffmann-La Roche Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use COLUMVI® safely and effectively. See full prescribing information for COLUMVI.
COLUMVI (glofitamab-gxbm) injection, for intravenous use
Initial U.S. Approval: 2023WARNING: CYTOKINE RELEASE SYNDROME
See full prescribing information for complete boxed warning
Cytokine Release Syndrome (CRS), including serious or fatal reactions, can occur in patients receiving COLUMVI. Premedicate before each dose, and initiate treatment with the COLUMVI step-up dosing schedule to reduce the risk of CRS. Withhold COLUMVI until CRS resolves or permanently discontinue based on severity. (2.1, 2.2, 2.3, 2.4, 5.1)
INDICATIONS AND USAGE
COLUMVI is a bispecific CD20-directed CD3 T-cell engager indicated for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma, not otherwise specified (DLBCL, NOS) or large B-cell lymphoma (LBCL) arising from follicular lymphoma, after two or more lines of systemic therapy.
This indication is approved under accelerated approval based on response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s). (1)
DOSAGE AND ADMINISTRATION
- Pretreat with a single 1,000 mg dose of obinutuzumab intravenously 7 days before initiation of COLUMVI (Cycle 1 Day 1). (2.2)
- Administer premedications as recommended. (2.3)
- Administer only as an intravenous infusion. (2.1)
- Recommended dosage (2.2):
Treatment Cycle* Day Dose of COLUMVI - * Cycle = 21 days
Day 1 Obinutuzumab 1,000 mg Cycle 1 Day 8 Step-up dose 1 2.5 mg Day 15 Step-up dose 2 10 mg Cycle 2 to 12 Day 1 30 mg - Administer in a facility equipped to monitor and manage CRS. (2.1, 2.2)
- Patients should be hospitalized for the 2.5 mg step-up dose and for subsequent infusions as recommended. (2.1, 2.2)
- See Full Prescribing Information for instructions on preparation and administration. (2.5, 2.6, 2.7)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Neurologic Toxicity: Can cause serious neurologic toxicity, including Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS). Monitor for neurologic toxicity; withhold or permanently discontinue based on severity. (5.2)
- Serious Infections: Can cause serious or fatal infections. Monitor patients for signs and symptoms of infection and treat appropriately. (5.3)
- Tumor Flare: Can cause serious tumor flare reactions. Monitor patients at risk for complications of tumor flare. (5.4)
- Embryo-Fetal Toxicity: May cause fetal harm. Advise females of reproductive potential of the potential risk to the fetus and to use effective contraception. (5.5, 8.1, 8.3)
ADVERSE REACTIONS
The most common (≥ 20%) adverse reactions, excluding laboratory abnormalities, are cytokine release syndrome, musculoskeletal pain, rash, and fatigue. The most common (≥ 20%) Grade 3 to 4 laboratory abnormalities are lymphocyte count decreased, phosphate decreased, neutrophil count decreased, uric acid increased, and fibrinogen decreased. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genentech at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CYTOKINE RELEASE SYNDROME
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
2.2 Recommended Dosage
2.3 Recommended Premedication and Prophylactic Medications
2.4 Dosage Modifications for Adverse Reactions
2.5 Preparation into an Intravenous Bag
2.6 Preparation of 2.5 mg Dose into an Intravenous Syringe
2.7 Storage and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome
5.2 Neurologic Toxicity
5.3 Serious Infections
5.4 Tumor Flare
5.5 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Relapsed or Refractory DLBCL, NOS or LBCL Arising from Follicular Lymphoma
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CYTOKINE RELEASE SYNDROME
Cytokine Release Syndrome (CRS), including serious or fatal reactions, can occur in patients receiving COLUMVI. Premedicate before each dose, and initiate treatment with the COLUMVI step-up dosing schedule to reduce the risk of CRS. Withhold COLUMVI until CRS resolves or permanently discontinue based on severity [see Dosage and Administration (2.1, 2.2, 2.3, and 2.4) and Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
COLUMVI is indicated for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma, not otherwise specified (DLBCL, NOS) or large B-cell lymphoma (LBCL) arising from follicular lymphoma, after two or more lines of systemic therapy.
This indication is approved under accelerated approval based on response rate and durability of response [see Clinical Studies (14.1)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
- Administer only as an intravenous infusion through a dedicated infusion line that includes a sterile 0.2-micron in-line filter.
- Administer COLUMVI diluted solution via intravenous bag infusion. The 2.5 mg dose may alternatively be administered via intravenous syringe infusion [see Dosage and Administration (2.5, 2.6, 2.7)].
- COLUMVI should only be administered by a healthcare professional with immediate access to appropriate medical support, including supportive medications to manage severe CRS [see Dosage and Administration (2.4)].
- Ensure adequate hydration before administering COLUMVI.
- Premedicate before each dose [see Dosage and Administration (2.3)].
- Following pretreatment with obinutuzumab, administer COLUMVI according to the step-up dosing schedule in Table 1 with appropriate premedication, including dexamethasone, to reduce the incidence and severity of CRS [see Dosage and Administration (2.3)].
- Due to the risk of CRS, patients should be hospitalized during and for 24 hours after completion of infusion of step-up dose 1 (2.5 mg on Cycle 1 Day 8) [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)].
- Patients who experienced any grade CRS during step-up dose 1 should be hospitalized during and for 24 hours after completion of step-up dose 2 (10 mg on Cycle 1 Day 15). CRS with step-up dose 2 can occur in patients who did not experience CRS with step-up dose 1 [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)].
- For subsequent doses, patients who experienced Grade ≥ 2 CRS with their previous infusion should be hospitalized during and for 24 hours after the completion of the next COLUMVI infusion.
2.2 Recommended Dosage
Pretreatment with Obinutuzumab
Pretreat all patients with a single 1,000 mg dose of obinutuzumab administered as an intravenous infusion on Cycle 1 Day 1, 7 days prior to initiation of COLUMVI (see Table 1) to deplete the circulating and lymphoid tissue B cells.
Obinutuzumab should be administered as an intravenous infusion at 50 mg/hour. The rate of infusion can be escalated in 50 mg/hour increments every 30 minutes to a maximum of 400 mg/hour. Refer to the obinutuzumab prescribing information for complete dosing information.
COLUMVI Step-up Dose Schedule
COLUMVI dosing begins with a step-up dose schedule. Following completion of pretreatment with obinutuzumab on Cycle 1 Day 1, administer COLUMVI as an intravenous infusion according to the step-up dose schedule in Table 1. Administer premedications for each dose of COLUMVI as described in Table 3 [see Dosage and Administration (2.3)].
Table 1: COLUMVI Dosing Schedule (21-Day Treatment Cycles) Treatment cycle Day Dose of COLUMVI Duration of infusion - * Refer to "Pretreatment with obinutuzumab" described above.
- † For patients who experience CRS with their previous dose of COLUMVI, the time of infusion may be extended up to 8 hours.
- ‡ If the patient experienced CRS with the previous dose, the duration of infusion should be maintained at 4 hours.
Cycle 1 Day 1 Obinutuzumab* Day 8 Step-up dose 1 2.5 mg 4 hours† Day 15 Step-up dose 2 10 mg Cycle 2 Day 1 30 mg 4 hours† Cycle 3 to 12 Day 1 30 mg 2 hours‡ Continue COLUMVI for a maximum of 12 cycles (inclusive of Cycle 1 step-up dosing) or until disease progression or unacceptable toxicity, whichever occurs first.
Monitoring for Cytokine Release Syndrome [see Warnings and Precautions (5.1)]
- Administer the COLUMVI infusions intravenously in a healthcare setting with immediate access to medical support to manage CRS, including severe CRS.
- For the first COLUMVI step-up dose (2.5 mg on Cycle 1 Day 8), patients should be hospitalized during and for 24 hours after completion of the COLUMVI infusion.
- Patients who experienced any grade CRS during step-up dose 1 should be hospitalized during and for 24 hours after completion of step-up dose 2 (10 mg on Cycle 1 Day 15). CRS with step-up dose 2 can occur in patients who did not experience CRS with step-up dose 1.
- For subsequent infusions (30 mg on Day 1 of Cycle 2 or subsequent cycles), patients who experienced Grade ≥ 2 CRS with their previous infusion should be hospitalized during and for 24 hours after completion of the next COLUMVI infusion.
- For monitoring after delayed or missed doses of COLUMVI, follow the recommendations in Table 2.
Delayed or Missed Doses
If a dose of COLUMVI is delayed, restart therapy based on the recommendations made in Table 2, then resume the treatment schedule accordingly.
For repeat of the 2.5 mg dose patients should be hospitalized during and for 24 hours after completion of the COLUMVI infusion. For the repeat of the 10 mg dose, patients should be hospitalized during and for 24 hours after completion of the COLUMVI infusion if any grade CRS occurred during the most recent 2.5 mg dose.
Table 2: Recommendations for Restarting COLUMVI After Dose Delay Last Dose Administered Time Since Last Dose Administered Action for Next Dose(s)* - * Administer premedication as per Table 3 for all patients.
- † Patients should be hospitalized during and for 24 hours after completing infusion of the 2.5 mg dose.
- ‡ Patients should be hospitalized during and for 24 hours after completing infusion of the 10 mg dose if CRS occurred during the most recent 2.5 mg dose.
Obinutuzumab pretreatment (Cycle 1 Day 1) ≤ 2 weeks - Administer COLUMVI 2.5 mg (Cycle 1 Day 8)†, then resume the planned treatment schedule.
> 2 weeks - Repeat obinutuzumab 1,000 mg pretreatment (Cycle 1 Day 1).
- Then administer COLUMVI 2.5 mg (Cycle 1 Day 8)† and resume the planned treatment schedule.
COLUMVI 2.5 mg
(Cycle 1 Day 8)≤ 2 weeks - Administer COLUMVI 10 mg (Cycle 1 Day 15)‡, then resume the planned treatment schedule.
> 2 to ≤ 4 weeks > 4 weeks COLUMVI 10 mg
(Cycle 1 Day 15)≤ 2 weeks - Administer COLUMVI 30 mg (Cycle 2 Day 1), then resume the planned treatment schedule.
> 2 to ≤ 6 weeks - Repeat COLUMVI 10 mg (Cycle 1 Day 15).‡
- Then administer COLUMVI 30 mg (Cycle 2 Day 1) and resume the planned treatment schedule.
> 6 weeks COLUMVI 30 mg
(Cycle 2 onwards)≤ 6 weeks - Administer COLUMVI 30 mg, then resume the planned treatment schedule.
> 6 weeks 2.3 Recommended Premedication and Prophylactic Medications
Premedication
Administer the following premedications to reduce the risk of CRS and infusion-related reactions [see Warnings and Precautions (5.1)].
Table 3: Premedications to be Administered for COLUMVI Infusion Day of Treatment Cycle Patients requiring premedication Premedication Administration - * If dexamethasone is not available, administer prednisone 100 mg, prednisolone 100 mg, or methylprednisolone 80 mg intravenously.
Cycle 1, Day 8 and Day 15; Cycle 2; Cycle 3 Dexamethasone 20 mg intravenously* Completed at least 1 hour prior to COLUMVI infusion. All patients Acetaminophen 500 mg to 1,000 mg orally At least 30 minutes before COLUMVI infusion. Antihistamine (diphenhydramine 50 mg orally or intravenously or equivalent) Completed at least 30 minutes before COLUMVI infusion. All subsequent infusions All patients Acetaminophen 500 mg to 1,000 mg orally At least 30 minutes before COLUMVI infusion. Antihistamine (diphenhydramine 50 mg orally or intravenously or equivalent) Completed at least 30 minutes before COLUMVI infusion. Patients who experienced any grade CRS with the previous dose Dexamethasone 20 mg intravenously* Completed at least 1 hour prior to COLUMVI infusion. Tumor Lysis Syndrome Prophylaxis
Before starting COLUMVI, administer anti-hyperuricemics to patients at risk of tumor lysis syndrome, ensure adequate hydration status, and monitor as appropriate [see Adverse Reactions (6.1)].
Infection Prophylaxis
Before starting COLUMVI, consider initiation of antiviral prophylaxis to prevent herpes virus reactivation. Consider prophylaxis for cytomegalovirus infection, pneumocystis jirovecii pneumonia (PJP), and other opportunistic infections in patients at increased risk [see Warnings and Precautions (5.3)].
2.4 Dosage Modifications for Adverse Reactions
No dosage reduction for COLUMVI is recommended.
Cytokine Release Syndrome
Identify CRS based on clinical presentation [see Warnings and Precautions (5.1)]. Evaluate for and treat other causes of fever, hypoxia, and hypotension.
If CRS is suspected, withhold COLUMVI and manage according to the recommendations in Table 4 and current practice guidelines. Administer supportive care for CRS, which may include intensive care for severe or life-threatening cases.
Table 4: Recommendations for Management of Cytokine Release Syndrome Grade* Presenting Symptoms Actions - * American Society for Transplantation and Cellular Therapy (ASTCT) 2019 consensus grading criteria.
- † Premedication may mask fever. Therefore, if clinical presentation is consistent with CRS, follow these management guidelines.
- ‡ Duration of infusion may be extended up to 8 hours, as appropriate for that cycle (see Table 1).
- § Refer to Table 2 for information on restarting COLUMVI after dose delays [see Dosage and Administration (2.2)].
- ¶ Low-flow oxygen defined as oxygen delivered at < 6 L/minute, high-flow oxygen defined as oxygen delivered at ≥ 6 L/minute.
Grade 1 Temperature ≥ 100.4°F (38°C)† Grade 2 Temperature ≥ 100.4°F (38°C)† with:
Hypotension not requiring vasopressors
and/or
Hypoxia requiring low-flow oxygen¶ by nasal cannula or blow-by- Withhold COLUMVI and manage per current practice guidelines.
- If symptoms resolve, restart infusion at a slower rate.‡
- Ensure CRS symptoms are resolved for at least 72 hours before next dose.§
- For the next dose, consider a slower infusion rate, monitor more frequently, and consider hospitalization.
- For recurrent Grade 2 CRS, manage per Grade 3 CRS.
Grade 3 Temperature ≥ 100.4°F (38°C)† with:
Hypotension requiring vasopressor (with or without vasopressin)
and/or
Hypoxia requiring high-flow oxygen¶ by nasal cannula, face mask, non-rebreather mask, or Venturi mask- Withhold COLUMVI and manage per current practice guidelines, which may include intensive care.
- Ensure CRS symptoms are resolved for at least 72 hours before next dose.§
- Hospitalize for the next dose, monitor more frequently, and consider a slower infusion rate.‡
- For recurrent Grade 3 CRS, permanently discontinue COLUMVI.
Grade 4 Temperature ≥ 100.4°F (38°C)† with:
Hypotension requiring multiple vasopressors (excluding vasopressin)
and/or
Hypoxia requiring oxygen by positive pressure (e.g., CPAP, BiPAP, intubation, and mechanical ventilation)- Permanently discontinue COLUMVI and manage per current practice guidelines, which may include intensive care.
Neurologic Toxicity, Including ICANS
Management recommendations for neurologic toxicity, including ICANS, is summarized in Table 5. At the first sign of neurologic toxicity, including ICANS, consider neurology evaluation and withholding COLUMVI based on the type and severity of neurotoxicity. Rule out other causes of neurologic symptoms. Provide supportive therapy, which may include intensive care.
Table 5: Recommended Dosage Modification for Neurologic Toxicity (Including ICANS) Adverse Reaction Severity*,† Actions - * Based on National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.03.
- † Based on ASTCT 2019 grading for ICANS.
- ‡ Consider the type of neurologic toxicity before deciding to withhold COLUMVI.
- § See Dosage and Administration (2.2) on restarting COLUMVI after dose delays.
- ¶ Evaluate benefit-risk before restarting COLUMVI.
Grade 1 - Continue COLUMVI and monitor neurologic toxicity symptoms.
- If ICANS, manage per current practice guidelines.
Grade 2 Neurologic Toxicity* (including ICANS†) [see Warnings and Precautions (5.2)] Grade 3 - Withhold COLUMVI until neurologic toxicity symptoms improve to Grade 1 or baseline for at least 7 days.§, ¶
- For Grade 3 neurologic events lasting more than 7 days, consider permanently discontinuing COLUMVI.
- Provide supportive therapy, and consider neurology evaluation.
- If ICANS, manage per current practice guidelines.
Grade 4 - Permanently discontinue COLUMVI.
- Provide supportive therapy, which may include intensive care, and consider neurology evaluation.
- If ICANS, manage per current practice guidelines.
Other Adverse Reactions
Table 6: Recommended Dosage Modifications for Other Adverse Reactions Adverse Reactions* Severity* Actions - * Based on NCI CTCAE, version 4.03.
- † See Dosage and Administration (2.2) on restarting COLUMVI after dose delays.
Infections [see Warnings and Precautions (5.3)] Grades 1 – 4 - Withhold COLUMVI in patients with active infection until the infection resolves.†
- For Grade 4, consider permanent discontinuation of COLUMVI.
Grade 1 - Monitor for signs and symptoms of compression or obstruction due to mass effect secondary to tumor flare.
Tumor flare [see Warnings and Precautions (5.4)] Grades 2 – 4 - Monitor for signs and symptoms of compression or obstruction due to mass effect secondary to tumor flare, and institute appropriate treatment including antihistamine and corticosteroids.
- Withhold COLUMVI until tumor flare resolves.†
Neutropenia Absolute neutrophil count less than 0.5 × 109/L - Withhold COLUMVI until absolute neutrophil count is 0.5 × 109/L or higher.†
Thrombocytopenia Platelet count less than 50 × 109/L - Withhold COLUMVI until platelet count is 50 × 109/L or higher.†
Other Adverse Reactions [see Adverse Reactions (6.1)] Grade 3 or higher - Withhold COLUMVI until the toxicity resolves to Grade 1 or baseline.†
2.5 Preparation into an Intravenous Bag
This section describes preparation of all doses of COLUMVI into an intravenous bag. For preparation instructions for the 2.5 mg dose into an intravenous syringe, see subsection 2.6 [see Dosage and Administration (2.6)].
Preparation
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. COLUMVI is a colorless clear solution. Discard the vial if the solution is cloudy, discolored, or contains visible particles.
- Use aseptic technique when preparing the COLUMVI diluted solution for intravenous infusion.
Dilution for Intravenous Bag Infusion
- Determine the dose, total volume of COLUMVI solution, and the number of COLUMVI vials needed (see Table 7).
-
Select an appropriate size infusion bag of 0.9% Sodium Chloride Injection or 0.45% Sodium Chloride Injection (see Table 7).
- COLUMVI diluted with 0.9% Sodium Chloride Injection is compatible with intravenous infusion bags composed of polyvinyl chloride (PVC), polyethylene (PE), polypropylene (PP) or polyolefin.
- COLUMVI diluted with 0.45% Sodium Chloride Injection is compatible with intravenous infusion bags composed of PVC.
- Prepare the infusion bag by withdrawing and discarding the volume from the infusion bag according to Table 7.
- Withdraw the required volume of COLUMVI from the vial(s) using a sterile needle and syringe and dilute into the infusion bag to a final concentration of 0.1 mg/mL to 0.6 mg/mL according to Table 7.
Table 7: Dilution of COLUMVI into an intravenous infusion bag Dose of COLUMVI Size of 0.9% Sodium Chloride Injection or 0.45% Sodium Chloride Injection infusion bag Volume to be withdrawn and discarded from the infusion bag Volume of COLUMVI to be added to the infusion bag Total volume to be infused 2.5 mg 50 mL 27.5 mL 2.5 mL 25 mL 10 mg 50 mL 10 mL 10 mL 50 mL 100 mL 10 mL 10 mL 100 mL 30 mg 50 mL 30 mL 30 mL 50 mL 100 mL 30 mL 30 mL 100 mL - Discard any unused COLUMVI left in the vial.
- Gently invert the infusion bag to mix the solution, in order to avoid excess foaming. Do not shake.
2.6 Preparation of 2.5 mg Dose into an Intravenous Syringe
This section describes the alternative method of preparation of the 2.5 mg dose of COLUMVI into an intravenous syringe. For preparation instructions for all doses into an intravenous infusion bag, see subsection 2.5 [see Dosage and Administration (2.5)].
Preparation
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. COLUMVI is a colorless clear solution. Discard the vial if the solution is cloudy, discolored, or contains visible particles.
- Use aseptic technique when preparing the COLUMVI diluted solution for intravenous syringe infusion.
Dilution for Intravenous Syringe Infusion (Alternative Method for 2.5 mg Dose Only)
- Draw 22.5 mL of 0.9% Sodium Chloride Injection or 0.45% Sodium Chloride Injection into a 30 mL syringe composed of PP (see Table 8).
- Withdraw 2.5 mL of COLUMVI from the vial using a sterile needle into a second syringe (see Table 8). Discard any unused COLUMVI left in the vial.
- Attach a connector to the two syringes and transfer COLUMVI into the 30 mL syringe. The final concentration of COLUMVI should be 0.1 mg/mL.
Table 8: Dilution of COLUMVI into an intravenous syringe Dose of COLUMVI Volume of 0.9% Sodium Chloride Injection or 0.45% Sodium Chloride Injection to be added to the syringe Volume of COLUMVI to be added to the syringe Total volume to be infused 2.5 mg 22.5 mL 2.5 mL 25 mL - Disconnect the syringes. Draw air into the syringe containing the COLUMVI diluted solution and close.
- Gently invert the syringe to mix the solution, in order to avoid excessive foaming. Do not shake.
- Remove air bubbles from the syringe before administration.
2.7 Storage and Administration
Storage of Diluted Product
Immediately use diluted COLUMVI solution. If not used immediately, the diluted solution can be stored:
- Refrigerated at 2°C to 8°C (36°F to 46°F) for up to 64 hours, or
- At room temperature up to 25°C (77°F) for up to 4 hours.
Do not freeze the diluted infusion solution.
Discard diluted infusion solution if storage time exceeds these limits.
COLUMVI Administration
- Administer COLUMVI as an intravenous infusion only through a dedicated infusion line that includes a sterile 0.2-micron in-line filter using an intravenous infusion pump or syringe pump. Prime the infusion line with the diluted infusion solution.
- No incompatibilities have been observed with infusion sets with product-contacting surfaces of polyurethane (PUR), PVC, PE, polybutadiene (PBD), polyetherurethane (PEU), polycarbonate (PC), silicone, polytetrafluoroethylene (PTFE), or acrylonitrile butadiene styrene (ABS), and in-line filter membranes composed of polyethersulfone (PES) or polysulfone.
- See Table 1 for duration of infusion. The maximum time for the administration of the diluted infusion solution may be extended up to 8 hours (see Table 4).
- To ensure the entire dose of COLUMVI is administered, replace the empty infusion bag or syringe with an infusion bag or syringe containing 0.9% Sodium Chloride Injection or 0.45% Sodium Chloride Injection connected to the same infusion line. Continue the infusion at the same rate until the recommended infusion duration is reached according to Table 1.
- Do not mix COLUMVI with other drugs.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome
COLUMVI can cause serious and fatal cytokine release syndrome (CRS) [see Adverse Reactions (6.1)].
Among 145 patients who received COLUMVI, CRS occurred in 70%, with Grade 1 CRS developing in 52% of all patients, Grade 2 in 14%, Grade 3 in 2.8%, and Grade 4 in 1.4%. The most common manifestations of CRS included fever, tachycardia, hypotension, chills, and hypoxia.
CRS occurred in 56% of patients after the 2.5 mg dose of COLUMVI, 35% after the 10 mg dose, 29% after the initial 30 mg target dose, and 2.8% after subsequent doses. With the first step-up dose of COLUMVI, the median time to onset of CRS (from the start of infusion) was 14 hours (range: 5 to 74 hours). CRS after any dose resolved in 98% of cases, with a median duration of CRS of 2 days (range: 1 to 14 days). Recurrent CRS occurred in 34% of all patients. CRS can first occur with the 10 mg dose; of 135 patients treated with the 10 mg dose of COLUMVI, 15 patients (11%) experienced their first CRS event with the 10 mg dose, of which 13 events were Grade 1, 1 event was Grade 2, and 1 event was Grade 3.
Administer COLUMVI in a facility equipped to monitor and manage CRS. Initiate therapy according to the COLUMVI step-up dosing schedule to reduce the risk of CRS, administer pretreatment medications, and ensure adequate hydration [Dosage and Administration (2.3)]. Patients should be hospitalized during and for 24 hours after completing infusion of the 2.5 mg step-up dose. Patients who experienced any grade CRS during the 2.5 mg step-up dose should be hospitalized during and for 24 hours after completion of the 10 mg step-up dose. For subsequent doses, patients who experienced Grade ≥ 2 CRS with the previous infusion should be hospitalized during and for 24 hours after the next COLUMVI infusion [see Dosage and Administration (2.1 and 2.2)].
At the first sign of CRS, immediately evaluate patients for hospitalization, manage per current practice guidelines, and administer supportive care; withhold or permanently discontinue COLUMVI based on severity [see Dosage and Administration (2.4)].
5.2 Neurologic Toxicity
COLUMVI can cause serious and fatal neurologic toxicity, including Immune Effector Cell-Associated Neurotoxicity (ICANS) [see Adverse Reactions (6.1)].
Among 145 patients who received COLUMVI, the most frequent neurologic toxicities of any grade were headache (10%), peripheral neuropathy (8%), dizziness or vertigo (7%), and mental status changes (4.8%, including confusional state, cognitive disorder, disorientation, somnolence, and delirium). Grade 3 or higher neurologic adverse reactions occurred in 2.1% of patients and included somnolence, delirium, and myelitis. Cases of ICANS of any grade occurred in 4.8% of patients.
Coadministration of COLUMVI with other products that cause dizziness or mental status changes may increase the risk of neurologic toxicity. Optimize concomitant medications and hydration to avoid dizziness or mental status changes. Institute fall precautions as appropriate.
Monitor patients for signs and symptoms of neurologic toxicity, evaluate, and provide supportive therapy; withhold or permanently discontinue COLUMVI based on severity [see Dosage and Administration (2.4)].
Evaluate patients who experience neurologic toxicity such as tremors, dizziness, or adverse reactions that may impair cognition or consciousness promptly, including potential neurology evaluation. Advise affected patients to refrain from driving and/or engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, until the neurologic toxicity fully resolves.
5.3 Serious Infections
COLUMVI can cause serious or fatal infections [see Adverse Reactions (6.1)].
Serious infections were reported in 16% of patients, including Grade 3 or 4 infections in 10%, and fatal infections in 4.8% of patients. Grade 3 or higher infections reported in ≥ 2% of patients were COVID-19 infection (6%), including COVID-19 pneumonia, and sepsis (4.1%). Febrile neutropenia occurred in 3.4% of patients.
COLUMVI should not be administered to patients with an active infection. Administer antimicrobial prophylaxis according to guidelines. Monitor patients before and during COLUMVI treatment for infection and treat appropriately. Withhold or consider permanent discontinuation of COLUMVI based on severity [see Dosage and Administration (2.4)].
5.4 Tumor Flare
COLUMVI can cause serious tumor flare [see Adverse Reactions (6.1)]. Manifestations include localized pain and swelling at the sites of the lymphoma lesions and/or dyspnea from new pleural effusions.
Tumor flare was reported in 12% of patients who received COLUMVI, including Grade 2 tumor flare in 4.8% of patients and Grade 3 tumor flare in 2.8%. Recurrent tumor flare occurred in two (12%) of the affected patients. Most tumor flare events occurred during Cycle 1, with a median time to first onset of 2 days (range: 1 to 16 days) after the first dose of COLUMVI. The median duration was 3.5 days (range: 1 to 35 days).
Patients with bulky tumors or disease located in close proximity to airways or a vital organ should be monitored closely during initial therapy. Monitor for signs and symptoms of compression or obstruction due to mass effect secondary to tumor flare, and institute appropriate treatment. Withhold COLUMVI until tumor flare resolves [see Dosage and Administration (2.4)].
5.5 Embryo-Fetal Toxicity
Based on its mechanism of action, COLUMVI may cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment with COLUMVI and for 1 month after the last dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Cytokine Release Syndrome [see Warnings and Precautions (5.1)]
- Neurologic Toxicity [see Warnings and Precautions (5.2)]
- Serious Infections [see Warnings and Precautions (5.3)]
- Tumor Flare [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Relapsed or Refractory DLBCL, NOS or LBCL Arising from Follicular Lymphoma
Study NP30179
The safety of COLUMVI was evaluated in Study NP30179, a multi-cohort, multicenter, single-arm clinical trial that included 154 adult patients with relapsed or refractory large B-cell lymphoma (LBCL) after two or more lines of systemic therapy [see Clinical Studies (14.1)]. The trial required an ECOG performance status of 0 or 1, absolute neutrophil count ≥ 1,500/µL, platelet count ≥ 75,000/µL independent of transfusion, serum creatinine ≤ 1.5 × upper limit of normal (ULN) or creatinine clearance (CLcr) ≥ 50 mL/min, and hepatic transaminases ≤ 3 × ULN. The trial excluded patients with active or previous central nervous system (CNS) lymphoma or CNS disease, acute infection, recent infection requiring intravenous antibiotics, or prior allogeneic hematopoietic stem cell transplantation (HSCT).
Patients received pretreatment with a single dose of obinutuzumab on Day 1 of Cycle 1 (seven days prior to start of COLUMVI). Following premedication, COLUMVI was administered by intravenous infusion according to the step-up dosing schedule with 2.5 mg on Day 8 of Cycle 1, and 10 mg on Day 15 of Cycle 1. Patients received the 30 mg COLUMVI dose by intravenous infusion on Day 1 of subsequent cycles for a maximum of 12 cycles (including step-up dosing). Each cycle was 21 days. Patients were hospitalized during and for 24 hours following completion of at least the first step-up dose.
Of the 154 patients who initiated study treatment, 145 received COLUMVI; nine patients (6%) did not receive COLUMVI due to infection, progressive disease, or patient decision. Patients received a median of 5 cycles of COLUMVI with 30% receiving all 12 cycles of COLUMVI.
Of patients who received COLUMVI, the median age was 66 years (range: 21 to 90 years); 66% were male; 77% were White, 4.8% were Asian, 1.4% were Black or African American, 6% were Hispanic or Latino. The main diagnoses were DLBCL, NOS and LBCL arising from follicular lymphoma.
Serious adverse reactions occurred in 48% of patients who received COLUMVI. Serious adverse reactions in ≥ 2% of patients included CRS, COVID-19 infection, sepsis, and tumor flare. Fatal adverse reactions occurred in 5% of patients from COVID-19 infection (3.4%), sepsis (1.4%), and delirium (0.6%).
Adverse reactions led to permanent discontinuation of COLUMVI in 7% of patients, including from infection, delirium, neutropenia, and CRS. Adverse reactions led to dose interruptions of COLUMVI in 19% of patients, most frequently (≥ 2%) from neutropenia and thrombocytopenia.
The most common (≥ 20%) adverse reactions, excluding laboratory terms, were CRS, musculoskeletal pain, rash, and fatigue. The most common Grade 3 to 4 laboratory abnormalities (≥ 20%) were lymphocyte count decreased, phosphate decreased, neutrophil count decreased, uric acid increased, and fibrinogen decreased.
Table 9 summarizes adverse reactions observed in Study NP30179.
Table 9: Select Adverse Reactions (≥ 10%) in Patients with Relapsed or Refractory LBCL Who Received COLUMVI in Study NP30179 Adverse Reactions COLUMVI
N=145All grades (%) Grade 3 or 4 (%) The table includes a combination of grouped and ungrouped terms. Adverse reactions were graded using NCI CTCAE version 4.03, with the exception of CRS, which was graded per ASTCT consensus criteria in most cases. - * Includes musculoskeletal pain, back pain, bone pain, flank pain, myalgia, neck pain, and pain in extremity.
- † Includes fatigue and asthenia.
- ‡ Includes edema, edema peripheral, swelling face, and face edema.
- § Includes rash, rash pruritic, rash maculo-papular, dermatitis, dermatitis acneiform, dermatitis exfoliative, erythema, palmar erythema, pruritus, and rash erythematous.
- ¶ Includes abdominal pain, abdominal discomfort, and abdominal pain upper.
Immune system disorders Cytokine release syndrome 70 4.1 Musculoskeletal and connective tissue disorders Musculoskeletal pain* 21 2.1 General disorders Fatigue† 20 1.4 Pyrexia 16 0 Edema‡ 10 0 Skin and subcutaneous tissue disorders Rash§ 20 1.4 Gastrointestinal disorders Constipation 14 0 Diarrhea 14 0 Nausea 10 0 Abdominal pain¶ 10 0 Neoplasms Tumor flare 12 2.8 Neurologic Disorders Headache 10 0 Clinically relevant adverse reactions occurring in < 10% of patients who received COLUMVI included infusion-related reaction, peripheral neuropathy, pneumonia, mental status changes, vomiting, tumor lysis syndrome, febrile neutropenia, upper respiratory tract infection, sepsis, herpes zoster infection, gastrointestinal hemorrhage, tremor, myelitis, and colitis.
Table 10 summarizes laboratory abnormalities in Study NP30179.
Table 10: Select Laboratory Abnormalities (≥ 20%) That Worsened from Baseline in Patients with Relapsed or Refractory LBCL Who Received COLUMVI in Study NP30179 Laboratory Abnormality COLUMVI* All Grades (%) Grade 3 or 4 (%) - * The denominator used to calculate the rate varied from 137 to 145 based on the number of patients with a baseline value and at least one post-treatment value.
- † Grade 4 neutrophil decrease occurred in 9% of patients.
Hematology Lymphocytes decreased 90 83 Hemoglobin decreased 72 8 Neutrophils decreased 56 26† Platelets decreased 56 8 Chemistry Fibrinogen decreased 84 21 Phosphate decreased 69 28 Sodium decreased 49 7 Calcium decreased 48 2.1 Gamma-glutamyl transferase increased 33 9 Potassium decreased 32 6 Uric acid increased 23 23 -
7 DRUG INTERACTIONS
For certain CYP substrates where minimal concentration changes may lead to serious adverse reactions, monitor for toxicities or drug concentrations of such CYP substrates when coadministered with COLUMVI.
Glofitamab-gxbm causes the release of cytokines [see Clinical Pharmacology (12.2)] that may suppress the activity of CYP enzymes, resulting in increased exposure of CYP substrates. Increased exposure of CYP substrates is more likely to occur after the first dose of COLUMVI on Cycle 1 Day 8 and up to 14 days after the first 30 mg dose on Cycle 2 Day 1 and during and after CRS [see Warnings and Precautions (5.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action COLUMVI may cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on the use of COLUMVI in pregnant women to evaluate for a drug-associated risk. No animal reproductive and developmental toxicity studies have been conducted with glofitamab-gxbm.
Glofitamab-gxbm causes T-cell activation and cytokine release; immune activation may compromise pregnancy maintenance. In addition, based on expression of CD20 on B cells and the finding of B-cell depletion in non-pregnant animals, glofitamab-gxbm can cause B-cell lymphocytopenia in infants exposed to glofitamab-gxbm in-utero. Human immunoglobulin G (IgG) is known to cross the placenta; therefore, COLUMVI has the potential to be transmitted from the mother to the developing fetus. Advise women of the potential risk to the fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of glofitamab-gxbm in human milk or the effects on the breastfed child or milk production. Because human IgG is present in human milk, and there is potential for glofitamab-gxbm absorption leading to B-cell depletion, advise women not to breastfeed during treatment with COLUMVI and for 1 month after the last dose of COLUMVI.
8.3 Females and Males of Reproductive Potential
COLUMVI may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating COLUMVI.
Contraception
Females
Advise female patients of reproductive potential to use effective contraception during treatment with COLUMVI and for 1 month after the last dose of COLUMVI [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and efficacy of COLUMVI in pediatric patients have not been established.
8.5 Geriatric Use
Of the 145 patients with relapsed or refractory LBCL who received COLUMVI in study NP30179, 55% were 65 years of age or older, and 23% were 75 years of age or older. There was a higher rate of fatal adverse reactions, primarily from COVID-19, in patients 65 years of age or older compared to younger patients [see Adverse Reactions (6.1)]. No overall differences in efficacy were observed between patients 65 years of age or older and younger patients.
-
11 DESCRIPTION
Glofitamab-gxbm is a bispecific CD20-directed CD3 T-cell engager. It is a recombinant humanized anti-CD20 anti-CD3ɛ bispecific immunoglobulin G1 (IgG1) monoclonal antibody produced in Chinese hamster ovary (CHO) cells. Glofitamab-gxbm has an approximate molecular weight of 197 kDa.
COLUMVI (glofitamab-gxbm) injection is a sterile, preservative-free, colorless, clear solution supplied in single-dose vials for intravenous infusion.
COLUMVI is supplied in 2.5 mg/2.5 mL and 10 mg/10 mL single-dose vials at a concentration of 1 mg/mL. Each mL of solution contains 1 mg glofitamab-gxbm, histidine (0.63 mg), histidine hydrochloride monohydrate (3.34 mg), methionine (1.49 mg), polysorbate 20 (0.5 mg), sucrose (82.15 mg), and Water for Injection, USP, at pH 5.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Glofitamab-gxbm is a bispecific antibody that binds to CD20 expressed on the surface of B cells, and to CD3 receptor expressed on the surface of T cells. Glofitamab-gxbm causes T-cell activation and proliferation, secretion of cytokines, and the lysis of CD20-expressing B cells. Glofitamab-gxbm showed anti-tumor activity in vivo in mouse models of DLBCL.
12.2 Pharmacodynamics
Circulating B Cell Count
Peripheral B cell counts decreased to undetectable levels (< 5 cells/microliter) in 86.5% of patients by Cycle 1 Day 7 after obinutuzumab pretreatment of 1,000 mg on Cycle 1 Day 1, and in 88.2% of patients by Cycle 1 Day 10 after the first glofitamab-gxbm dose of 2.5 mg on Cycle 1 Day 8.
Cytokine Concentrations
Plasma concentrations of cytokines (IL-2, IL-6, IL-10, TNF-α, and IFN-γ) were measured and transient elevation of cytokines was observed at doses of 0.045 mg and above. After administration of the recommended dosage of COLUMVI, the highest elevation of cytokines was generally observed within 6 hours after the first glofitamab-gxbm dose of 2.5 mg on Cycle 1 Day 8. The elevated cytokine levels generally returned to baseline within 48 hours after the first 30 mg dose on Cycle 2 Day 1.
12.3 Pharmacokinetics
The pharmacokinetics of glofitamab-gxbm was determined following pretreatment with a single dose of obinutuzumab of 1,000 mg and the pharmacokinetic parameters are presented as geometric mean (CV%) unless otherwise specified. Glofitamab-gxbm exposure increased dose-proportionally over the dose range from 0.005 to 30 mg (0.000167 to 1 time the recommended treatment dosage). Glofitamab-gxbm exposure parameters are summarized in Table 11 for the approved recommended dosage of COLUMVI.
Table 11: Exposure Parameters of Glofitamab-gxbm Following Pretreatment with a Single Dose of Obinutuzumab of 1,000 mg AUCtau (day∙mcg/mL) Cmax (mcg/mL) Ctrough (mcg/mL) Data presented as geometric mean (CV%). AUCtau = area under the concentration-time curve over one 21-day cycle; Cmax = maximum glofitamab-gxbm concentration; Ctrough = glofitamab-gxbm concentration prior to next dose; CV = geometric coefficient of variation. - * Steady state values are approximated at Cycle 6 (week 18).
First full 30 mg dose 44.5 (55%) 9.41 (27%) 0.52 (83%) Steady state* 30 mg dose 48.6 (33%) 9.44 (26%) 0.59 (67%) Elimination
At steady state, the glofitamab-gxbm terminal half-life is 7.6 days (24%) and the clearance is 0.617 L/day (33%).
Specific Populations
No clinically significant changes in the pharmacokinetics of glofitamab-gxbm were observed based on age (21 to 90 years), body weight (31 to 148 kg), sex, mild to moderate renal impairment (CLcr 30 to < 90 mL/min as estimated by Cockcroft-Gault formula) and mild hepatic impairment (total bilirubin > ULN to ≤ 1.5 × ULN or AST > ULN).
The effects of severe renal impairment (CLcr 15 to < 30 mL/min), end-stage renal disease (CLcr < 15 mL/min), or moderate to severe hepatic impairment (total bilirubin > 1.5 × ULN and any AST), and race/ethnicity on the pharmacokinetics of glofitamab-gxbm are unknown.
12.6 Immunogenicity
The observed incidence of antidrug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the study described below with the incidence of ADA in other studies, including those of glofitamab-gxbm.
During treatment in Study NP30179 (up to 9 months) [see Clinical Studies (14.1)], using an enzyme-linked immunosorbent assay (ELISA), the incidence of anti-glofitamab antibody formation was 1.1% (5/448) in patients treated with COLUMVI. Because of the low occurrence of ADAs, the effect of these antibodies on the pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of glofitamab-gxbm is unknown.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Relapsed or Refractory DLBCL, NOS or LBCL Arising from Follicular Lymphoma
The efficacy of COLUMVI was evaluated in Study NP30179 (NCT03075696), an open-label, multicenter, multicohort, single-arm clinical trial that included patients with relapsed or refractory LBCL after two or more lines of systemic therapy. The trial required an ECOG performance status of 0 or 1, absolute neutrophil count ≥ 1,500/µL, platelet count ≥ 75,000/µL independent of transfusion, serum creatinine ≤ 1.5 × ULN or CLcr ≥ 50 mL/min, and hepatic transaminases ≤ 3 × ULN. The trial excluded patients with active or previous CNS lymphoma or CNS disease, acute infection, recent infection requiring intravenous antibiotics, or prior allogeneic HSCT.
Following pretreatment with obinutuzumab on Cycle 1 Day 1, patients received COLUMVI by intravenous infusion, starting with a 2.5 mg step-up dose on Cycle 1 Day 8, followed by a 10 mg step-up dose on Cycle 1 Day 15, then 30 mg on Cycle 2 Day 1 and on Day 1 of each subsequent cycle. The cycle length was 21 days. COLUMVI was administered for up to 12 cycles unless patients experienced progressive disease or unacceptable toxicity.
The efficacy population consists of 132 patients with de novo DLBCL, NOS (80%) or LBCL arising from follicular lymphoma (20%) who received at least one dose of COLUMVI. The median age was 67 years (range: 21 to 90 years), 64% were male, 77% were White, 4.5% were Asian, 0.8% were Black or African American, 5% were Hispanic or Latino. The median number of prior lines of systemic therapy was 3 (range: 2 to 7). Most patients (83%) had refractory disease to the last therapy, 55% had primary refractory disease, 30% had received CAR-T cell therapy, and 19% had received autologous HSCT.
Efficacy was based on objective response rate (ORR) and duration of response (DOR), as determined by an Independent Review Committee (IRC) using the 2014 Lugano criteria.
Efficacy results are summarized in Table 12. The median time to first response was 42 days (range: 31 to 178 days). Among responders, the estimated median follow-up for DOR was 11.6 months.
Table 12: IRC-Assessed Efficacy in Patients with Relapsed or Refractory DLBCL, NOS or LBCL Arising from Follicular Lymphoma Outcome per IRC COLUMVI
N=132CI = confidence interval; NE = not estimable - * From date of first response (PR or CR) until disease progression or death due to any cause.
- † Kaplan-Meier estimate.
Overall Response Rate, n (%) 74 (56) (95% CI) (47, 65) Complete Response, n (%) 57 (43) (95% CI) (35, 52) Partial Response, n (%) 17 (13) (95% CI) (8, 20) Duration of Response* N = 74 Median DOR, months (95% CI)† 18.4 (11.4, NE) 9-month estimate, % (95% CI)† 68.5 (56.7, 80.3) -
16 HOW SUPPLIED/STORAGE AND HANDLING
COLUMVI (glofitamab-gxbm) injection is a sterile, preservative-free, colorless, clear solution for intravenous infusion.
COLUMVI is supplied as:
Carton Contents NDC One 2.5 mg/2.5 mL (1 mg/mL) single-dose vial NDC: 50242-125-01 One 10 mg/10 mL (1 mg/mL) single-dose vial NDC: 50242-127-01 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Cytokine Release Syndrome
Inform patients of the risk of CRS. Advise patients to seek immediate medical attention if they experience signs and symptoms of CRS (fever, hypoxia, hypotension, chills and tachycardia) [see Warnings and Precautions (5.1)].
Provide patients with the Patient Wallet Card that they should carry with them at all times. This card describes symptoms of CRS which, if experienced, should prompt the patient to seek immediate medical attention.
Neurologic Toxicity
Discuss the signs and symptoms associated with neurologic toxicity, including ICANS, headache, peripheral neuropathy, dizziness, or mental status changes. Advise patients to immediately contact their healthcare provider if they experience any signs or symptoms of neurologic toxicity. Advise patients who experience neurologic toxicity that impairs consciousness to refrain from driving or operating heavy or potentially dangerous machinery until neurologic toxicity resolves [see Warnings and Precautions (5.2)].
Serious Infections
Advise patients that COLUMVI can cause serious infections. Advise patients to notify their healthcare provider immediately if they develop any signs of infection (e.g., fever, chills, weakness) [see Warnings and Precautions (5.3)].
Tumor Flare
Inform patients of the potential risk of tumor flare reaction and to report any signs and symptoms associated with this event (e.g., localized pain and swelling) to their healthcare provider for evaluation [see Warnings and Precautions (5.4)].
Embryo-Fetal Toxicity
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider if they are pregnant or become pregnant. Advise females of reproductive potential to use effective contraception during treatment with COLUMVI and for 1 month after the last dose [see Warnings and Precautions (5.5) and Use in Specific Populations (8.1, 8.3)].
Advise women not to breastfeed while receiving treatment with COLUMVI and for 1 month after the last dose [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
MEDICATION GUIDE
COLUMVI® (ko-loom-vee)
(glofitamab-gxbm)
injection, for intravenous infusionThis Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 6/2025 What is the most important information I should know about COLUMVI?
COLUMVI can cause Cytokine Release Syndrome (CRS), a serious side effect that is common during treatment with COLUMVI, and can also be serious and lead to death.- Call your healthcare provider or get emergency medical help right away if you develop any signs or symptoms of CRS, including:
- fever of 100.4°F (38°C) or higher
- chills or shaking
- fast or irregular heartbeat
- dizziness or light-headedness
- trouble breathing
- shortness of breath
-
Due to the risk of CRS, you will receive COLUMVI on a "step-up dosing schedule".
- A single dose of a medicine called obinutuzumab will be given to you on the first day of your first treatment cycle (Day 1 of Cycle 1).
- You will start the COLUMVI step-up dosing schedule a week after the obinutuzumab dose. The step-up dosing schedule is when you receive smaller "step-up" doses of COLUMVI on Day 8 and Day 15 of Cycle 1. This is to help reduce your risk of CRS. You should be hospitalized during your infusion and for 24 hours after receiving the first step-up dose on Day 8. You should be hospitalized during your infusion and for 24 hours after receiving the second step-up dose on Day 15 if you experienced CRS during the first step-up dose.
- You will receive your first full dose of COLUMVI a week after the second step-up dose (this will be Day 1 of Cycle 2).
- If your dose of COLUMVI is delayed for any reason, you may need to repeat the "step-up dosing schedule".
- If you had more than mild CRS with your previous dose of COLUMVI, you should be hospitalized during and for 24 hours after receiving your next dose of COLUMVI.
- Before each dose of COLUMVI, you will receive medicines to help reduce your risk of CRS and infusion-related reactions.
- See "How will I receive COLUMVI?" for more information about how you will receive COLUMVI.
- Your healthcare provider will monitor you for CRS during treatment with COLUMVI and may treat you in a hospital if you develop signs and symptoms of CRS. Your healthcare provider may temporarily stop or completely stop your treatment with COLUMVI if you have severe side effects.
- Carry the COLUMVI Patient Wallet Card with you at all times and show it to all of your healthcare providers. The COLUMVI Patient Wallet Card lists the signs and symptoms of CRS you should get emergency medical help for right away.
What is COLUMVI?
COLUMVI is a prescription medicine used to treat adults with certain types of diffuse large B-cell lymphoma (DLBCL) or large B-cell lymphoma (LBCL) that has come back (relapsed) or that did not respond to previous treatment (refractory), and who have received 2 or more prior treatments for their cancer.
It is not known if COLUMVI is safe and effective in children.Before receiving COLUMVI, tell your healthcare provider about all of your medical conditions, including if you: - have an infection
- have kidney problems
- are pregnant or plan to become pregnant. COLUMVI may harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider should do a pregnancy test before you start treatment with COLUMVI.
- You should use effective birth control (contraception) during treatment and for 1 month after your last dose of COLUMVI. Talk to your healthcare provider about what birth control method is right for you during this time.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with COLUMVI.
- are breastfeeding or plan to breastfeed. It is not known if COLUMVI passes into your breastmilk. Do not breastfeed during treatment and for 1 month after your last dose of COLUMVI.
- COLUMVI will be given to you by your healthcare provider by infusion through a needle placed in your vein (intravenous infusion).
- Your COLUMVI treatment schedule is divided into cycles that are 21 days (3 weeks) long.
- On Day 1 of Cycle 1, your healthcare provider will give you a single dose of a medicine called obinutuzumab by intravenous infusion. You will then receive COLUMVI on Day 8 and Day 15 of Cycle 1. Starting with Cycle 2, you will receive COLUMVI 1 time every three weeks.
What should I avoid while receiving COLUMVI?
Do not drive, operate heavy machinery, or do other dangerous activities if you develop dizziness, confusion, shaking (tremors), sleepiness, or any other symptoms that impair consciousness until your signs and symptoms go away. These may be signs and symptoms of neurologic problems.
See "What are the possible side effects of COLUMVI?" for more information about signs and symptoms of neurologic problems.What are the possible side effects of COLUMVI?
COLUMVI may cause serious side effects, including:- Cytokine Release Syndrome. See "What is the most important information I should know about COLUMVI?"
- Neurologic problems. COLUMVI can cause serious neurologic problems that may lead to death. Your healthcare provider will monitor you for neurologic problems during treatment with COLUMVI. Your healthcare provider may also refer you to a healthcare provider who specializes in neurologic problems. Tell your healthcare provider right away if you develop any signs or symptoms of neurologic problems, including:
- headache
- confusion and disorientation
- difficulty paying attention or understanding things
- trouble speaking
- sleepiness
- memory problems
- numbness, tingling, or weakness of the hands or feet
- dizziness
- shaking (tremors)
- Serious infections. COLUMVI can cause serious infections that may lead to death. Your healthcare provider will monitor you for signs and symptoms of infection and treat you as needed. Tell your healthcare provider right away if you develop any signs of infection, including: fever, chills, weakness, cough, shortness of breath, or sore throat.
-
Growth in your tumor or worsening of tumor related problems (tumor flare). Tell your healthcare provider if you get any of these signs or symptoms of tumor flare:
- tender or swollen lymph nodes
- pain or swelling at the site of the tumor
- chest pain
- cough
- trouble breathing
Your healthcare provider may temporarily stop or completely stop treatment with COLUMVI if you develop certain side effects.
The most common side effects of COLUMVI include: CRS, muscle and bone pain, rash, and tiredness.
The most common severe abnormal lab test results with COLUMVI include: decreased white blood cells, decreased phosphate (an electrolyte), increased uric acid levels, and decreased fibrinogen (a protein that helps with blood clotting).
These are not all the possible side effects of COLUMVI.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of COLUMVI.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your healthcare provider or pharmacist for information about COLUMVI that is written for health professionals.What are the ingredients in COLUMVI?
Active ingredient: glofitamab-gxbm
Inactive ingredients: histidine, histidine hydrochloride monohydrate, methionine, polysorbate 20, sucrose, and Water for injection.
Manufactured by: Genentech, Inc., A Member of the Roche Group, 1 DNA Way, South San Francisco, CA 94080-4990
U.S. License No.: 1048
For more information, go to www.COLUMVI.com or call 1-877-436-3683. -
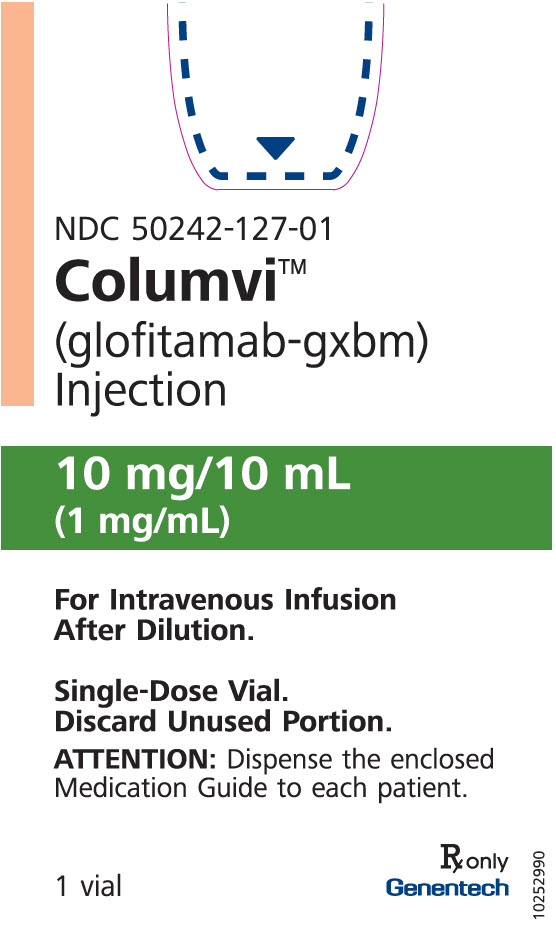
PRINCIPAL DISPLAY PANEL - 10 mg/10 mL Vial Carton
NDC: 50242-127-01
Columvi®
(glofitamab-gxbm)
Injection10 mg/10 mL
(1 mg/mL)For Intravenous Infusion
After Dilution.Single-Dose Vial.
Discard Unused Portion.ATTENTION: Dispense the enclosed
Medication Guide to each patient.1 vial
Rx only
Genentech11038205
-
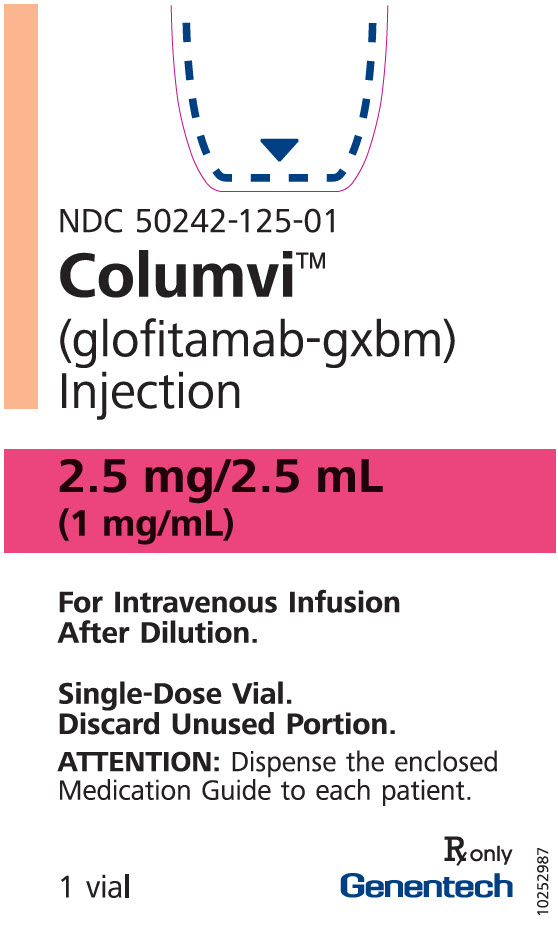
PRINCIPAL DISPLAY PANEL - 2.5 mg/2.5 mL Vial Carton
NDC: 50242-125-01
Columvi®
(glofitamab-gxbm)
Injection2.5 mg/2.5 mL
(1 mg/mL)For Intravenous Infusion
After Dilution.Single-Dose Vial.
Discard Unused Portion.ATTENTION: Dispense the enclosed
Medication Guide to each patient.1 vial
Rx only
Genentech11038250
-
INGREDIENTS AND APPEARANCE
COLUMVI
glofitamab concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50242-127 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLOFITAMAB (UNII: 06P3KLK2J8) (GLOFITAMAB - UNII:06P3KLK2J8) GLOFITAMAB 10 mg in 10 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 6.3 mg in 10 mL HISTIDINE HYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) 33.4 mg in 10 mL METHIONINE (UNII: AE28F7PNPL) 14.9 mg in 10 mL SUCROSE (UNII: C151H8M554) 821.5 mg in 10 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 5 mg in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50242-127-01 1 in 1 CARTON 06/15/2023 1 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761309 06/15/2023 COLUMVI
glofitamab solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50242-125 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLOFITAMAB (UNII: 06P3KLK2J8) (GLOFITAMAB - UNII:06P3KLK2J8) GLOFITAMAB 2.5 mg in 2.5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 1.6 mg in 2.5 mL HISTIDINE HYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) 8.4 mg in 2.5 mL METHIONINE (UNII: AE28F7PNPL) 3.7 mg in 2.5 mL SUCROSE (UNII: C151H8M554) 205.4 mg in 2.5 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 1.25 mg in 2.5 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50242-125-01 1 in 1 CARTON 06/15/2023 1 2.5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761309 06/15/2023 Labeler - Genentech, Inc. (080129000) Registrant - Genentech, Inc. (080129000) Establishment Name Address ID/FEI Business Operations Roche Diagnostics 323105205 ANALYSIS(50242-125, 50242-127) , API MANUFACTURE(50242-125, 50242-127) Establishment Name Address ID/FEI Business Operations Genentech, Inc. (Oceanside) 146373191 ANALYSIS(50242-125, 50242-127) Establishment Name Address ID/FEI Business Operations Roche Diagnostics GmbH 315028860 ANALYSIS(50242-125, 50242-127) , MANUFACTURE(50242-125, 50242-127) , PACK(50242-125, 50242-127) , LABEL(50242-125, 50242-127) Establishment Name Address ID/FEI Business Operations F. Hoffmann-La Roche Ltd. 485244961 LABEL(50242-125, 50242-127) , PACK(50242-125, 50242-127)
Trademark Results [Columvi]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COLUMVI 97888709 not registered Live/Pending |
Genentech, Inc. 2023-04-14 |
 COLUMVI 88925128 not registered Live/Pending |
Genentech, Inc. 2020-05-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.