QUICK NUMB- lidocaine cream
Quick Numb by
Drug Labeling and Warnings
Quick Numb by is a Otc medication manufactured, distributed, or labeled by Clinical Resolution Laboratory, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
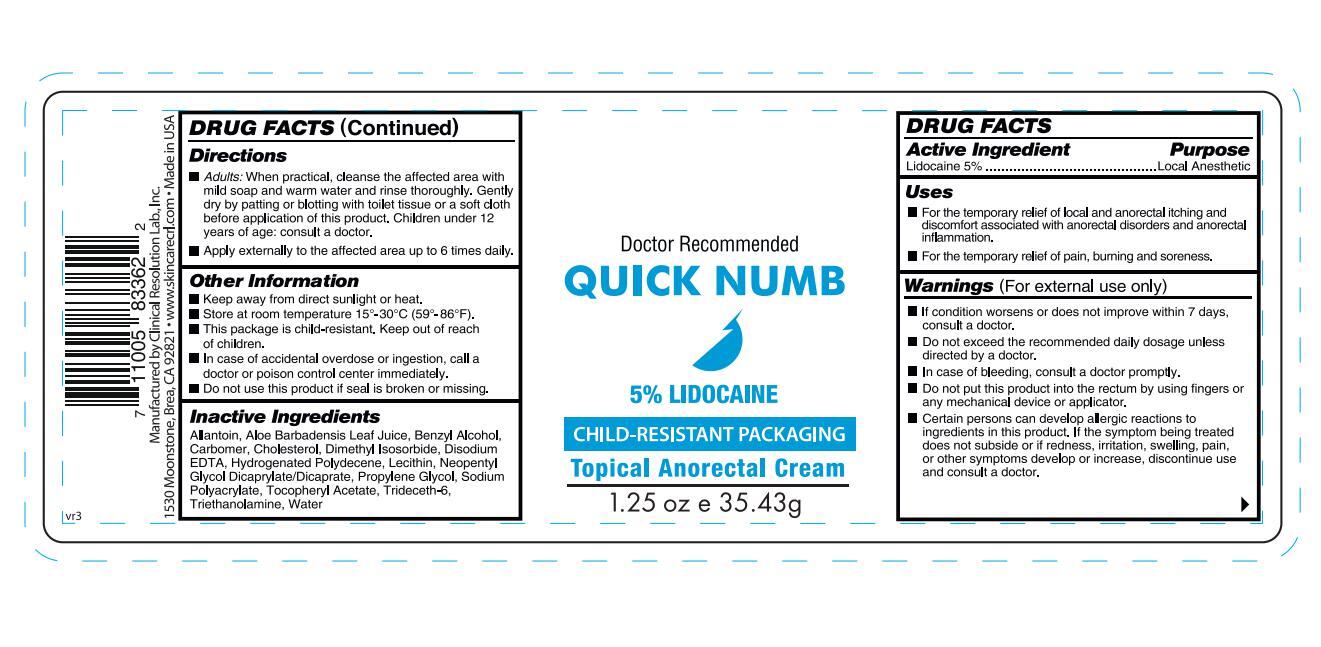
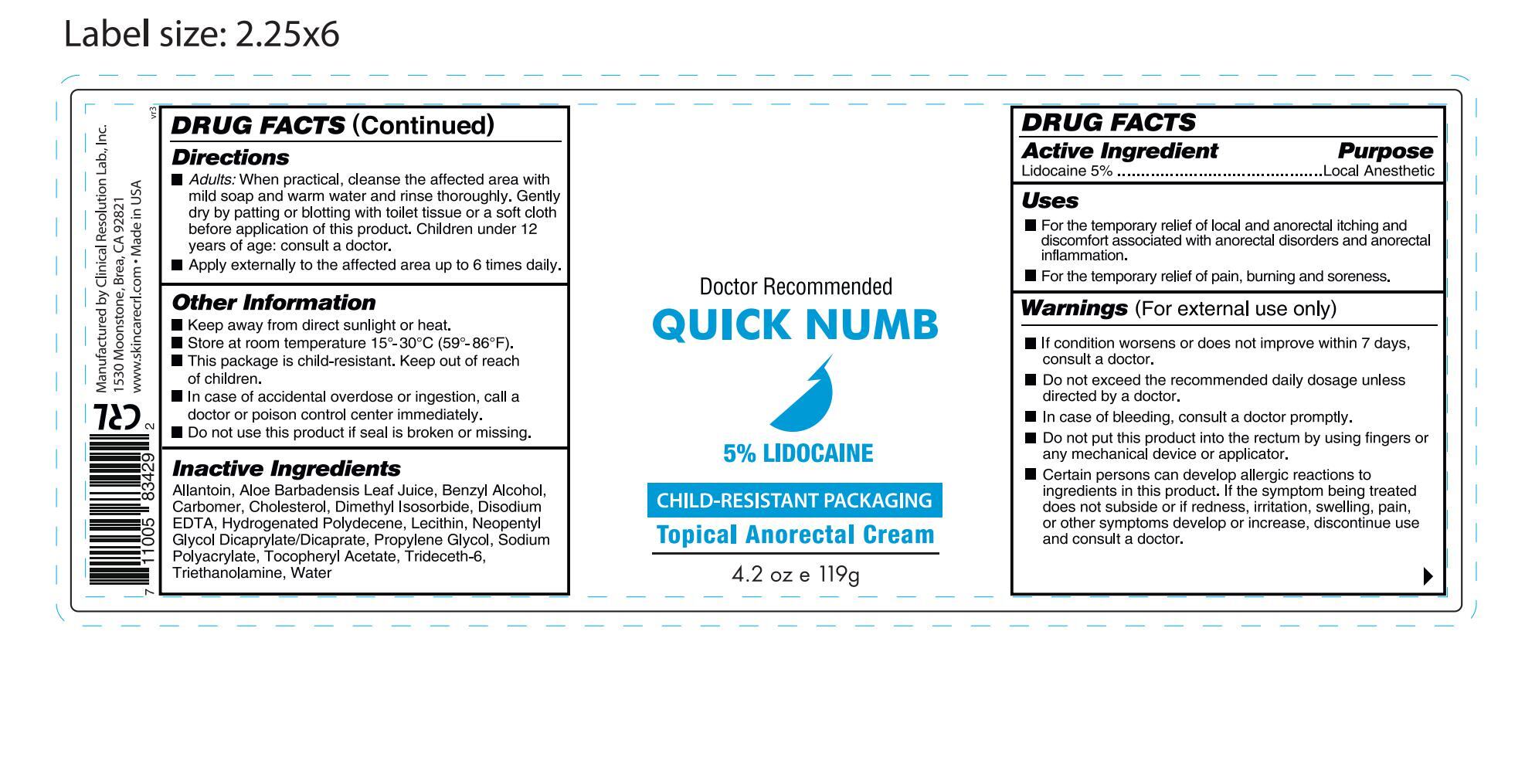
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
-
WARNINGS
Warnings (For external use only)
■ If condition worsens or does not improve within 7 days, consult a doctor.
■ Do not exceed the recommended daily dosage unless directed by a doctor.
■ In case of bleeding, consult a doctor promptly.
■ Do not put this product into the rectum by using fingers or any mechanical device or applicator.
■ Certain persons can develop allergic reactions to ingredients in this product. If the symptom being treated does not subside or if redness, irritation, swelling, pain, or other symptoms develop or increase, discontinue use and consult a doctor.
-
DOSAGE & ADMINISTRATION
Directions
■ Adults: When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product. Children under 12 years of age: consult a doctor.
■ Apply externally to the affected area up to 6 times daily.
-
KEEP OUT OF REACH OF CHILDREN
Other Information
■ Keep away from direct sunlight or heat.
■ Store at room temperature 15°-30°C (59°-86°F).
■ This package is child-resistant. Keep out of reach of children.
■ In case of accidental overdose or ingestion, call a doctor or poison control center immediately.
■ Do not use this product if seal is broken or missing.
-
INACTIVE INGREDIENT
Inactive Ingredients
Allantoin, Aloe Barbadensis Leaf Juice, Benzyl Alcohol, Carbomer, Cholesterol, Dimethyl Isosorbide, Disodium EDTA, Hydrogenated Polydecene, Lecithin, Neopentyl Glycol Dicaprylate/Dicaprate, Propylene Glycol, Sodium Polyacrylate, Tocopheryl Acetate, Trideceth-6, Triethanolamine, Water
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
QUICK NUMB
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63742-333 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) TRIDECETH-6 (UNII: 3T5PCR2H0C) CARBOMER (UNII: 0A5MM307FC) HYDROGENATED POLYDECENE (1500 CST) (UNII: 4YI0729529) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) CHOLESTEROL (UNII: 97C5T2UQ7J) ALOE BARBADENSIS LEAF JUICE (UNII: RUE8E6T4NB) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) BENZYL ALCOHOL (UNII: LKG8494WBH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALLANTOIN (UNII: 344S277G0Z) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) TRIETHANOLAMINE (UNII: 9O3K93S3TK) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63742-333-02 119 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/14/2025 2 NDC: 63742-333-01 35.43 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/14/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 05/14/2025 Labeler - Clinical Resolution Laboratory, Inc. (825047942) Establishment Name Address ID/FEI Business Operations Clinical Resolution Laboratory, Inc. 825047942 manufacture(63742-333)
Trademark Results [Quick Numb]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 QUICK NUMB 88135066 5693867 Live/Registered |
Lee, Justin 2018-09-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.