POTASSIUM PHOSPHATES injection, solution
Potassium Phosphates by
Drug Labeling and Warnings
Potassium Phosphates by is a Prescription medication manufactured, distributed, or labeled by Fresenius Kabi USA, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use POTASSIUM PHOSPHATES IN SODIUM CHLORIDE INJECTION safely and effectively. See full prescribing information for POTASSIUM PHOSPHATES IN SODIUM CHLORIDE INJECTION.
POTASSIUM PHOSPHATES IN SODIUM CHLORIDE injection, for intravenous use.
Initial U.S. Approval: 1983RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Potassium Phosphates in Sodium Chloride Injection is a phosphorus replacement product indicated as a source of phosphorus to correct hypophosphatemia in adults and pediatric patients who weigh 40 kg or greater when oral or enteral replacement is not possible, insufficient, or contraindicated. (1)
DOSAGE AND ADMINISTRATION
Important Preparation Instructions
- Do NOT dilute prior to administration. (2.1)
- Use this potassium phosphates in sodium chloride injection product only in patients who require the entire 15 mmoL phosphorus dose (potassium 22 mEq) and not any fraction thereof. If the entire 15 mmol phosphorus dose is not required, consider an alternative formulation of potassium phosphate. (2.1)
Important Administration Instructions
- Potassium Phosphates in Sodium Chloride Injection is only for administration to a patient with a serum potassium concentration less than 4 mEq/dL; otherwise, use an alternative source of phosphorus. (2.2)
-
This product contains phosphorus 15 mmol and potassium 22 mEq. (2.2)
- o The 100 mL ready-to-use container is for intravenous infusion into a central vein.
- o The 250 mL ready-to-use container is for intravenous infusion into a central or peripheral vein.
Recommended Dosage
- See full prescribing information for recommendations on initial or single dosing, repeated dosing, concentration and infusion rate. (2.3)
- Monitor serum phosphorus, potassium, calcium, and magnesium concentrations. (2.3)
- Patients with moderate renal impairment should start at the low end of the dosage range. Potassium Phosphates in Sodium Chloride Injection is contraindicated in patients with severe renal impairment. (2.4, 4)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Serious Cardiac Adverse Reactions with Bolus, or Rapid Intravenous Administration: Do not exceed the recommended infusion rate. Continuous electrocardiographic (ECG) monitoring may be needed during infusion. (2.3, 5.1)
- Hyperkalemia: Increased risk in patients with renal impairment, severe adrenal insufficiency, or treated with drugs that increase potassium. Patients with cardiac disease may be more susceptible. Do not exceed the maximum daily amount of potassium or the recommended infusion rate. Continuous ECG monitoring may be needed during infusion. (5.2, 7.1)
- Pulmonary Embolism due to Pulmonary Vascular Precipitates: If signs of pulmonary distress occur, stop the infusion and initiate a medical evaluation. (5.3)
- Hyperphosphatemia and Hypocalcemia: Monitor serum phosphorus and calcium concentrations during and following infusion. (5.4)
- Hypomagnesemia: Reported in patients with hypercalcemia and diabetic ketoacidosis. Monitor serum magnesium concentrations during treatment. (5.5)
- Vein Damage and Thrombosis: Infuse hypertonic solutions through a central catheter. (2.1, 5.6)
ADVERSE REACTIONS
Adverse reactions include hyperkalemia, hyperphosphatemia, hypocalcemia, and hypomagnesemia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation Instructions
2.2 Important Administration Instructions
2.3 Recommended Dosage
2.4 Recommended Dosage in Patients with Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Cardiac Adverse Reactions with Bolus or Rapid Intravenous Administration
5.2 Hyperkalemia
5.3 Pulmonary Embolism due to Pulmonary Vascular Precipitates
5.4 Hyperphosphatemia and Hypocalcemia
5.5 Hypomagnesemia
5.6 Vein Damage and Thrombosis
5.7 Laboratory Monitoring
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Other Products that Increase Serum Potassium
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation Instructions
- Potassium Phosphates in Sodium Chloride Injection is for intravenous infusion into a central or peripheral vein. Do NOT dilute prior to administration.
- Use this potassium phosphates in sodium chloride injection product only in patients who require the entire 15 mmoL phosphorus dose (potassium 22 mEq) and not any fraction thereof.
- If a dose of potassium phosphate is required that does not equal 15 mmoL of Potassium phosphates in Sodium Chloride Injection, then an alternative formulation of potassium phosphates should be considered.
- Visually inspect the solution for particulate matter and discoloration prior to administration. Do not administer unless solution is clear, and the seal of the container is intact.
- Always inspect the solution container before and after removal from the overwrap.
- Place the solution container on a clean, flat surface. Remove the solution container from the overwrap.
- Check the solution container for leaks by squeezing firmly. Discard if leaks are found.
- Immediately before inserting the infusion set, remove the twist-off infusion port.
- Use a non-vented infusion set or close the air-inlet on a vented set.
- Close the roller clamp of the infusion set.
- Hold the base of the twist-off infusion port, twist and push the spike until fully inserted. The infusion port is not intended to be spiked more than once.
- Suspend the solution container from the hanger hole.
- For single-dose only. Discard any unused portion.
2.2 Important Administration Instructions
- Check serum potassium and calcium concentrations prior to administration. Normalize the calcium before administering Potassium Phosphates in Sodium Chloride Injection [see Contraindications (4), Warnings and Precautions (5.3,5.4)].
- Potassium Phosphates in Sodium Chloride Injection is only for administration to a patient with a serum potassium concentration less than 4 mEq/dL [see Warnings and Precautions (5.3)]. If the potassium concentration is 4 mEq/dL or more, use an alternative source of phosphorus.
- Do not infuse with calcium-containing intravenous fluids [see Warnings and Precautions (5.4)].
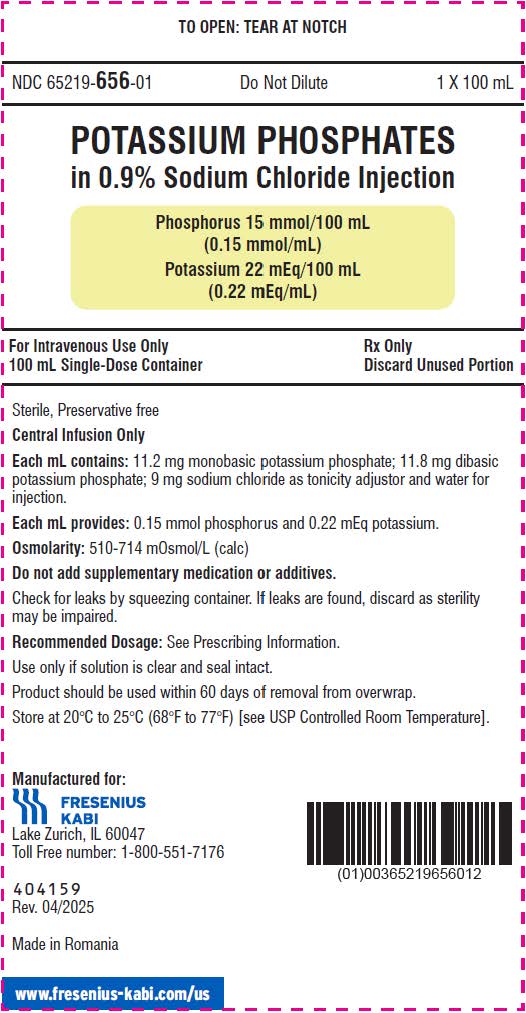
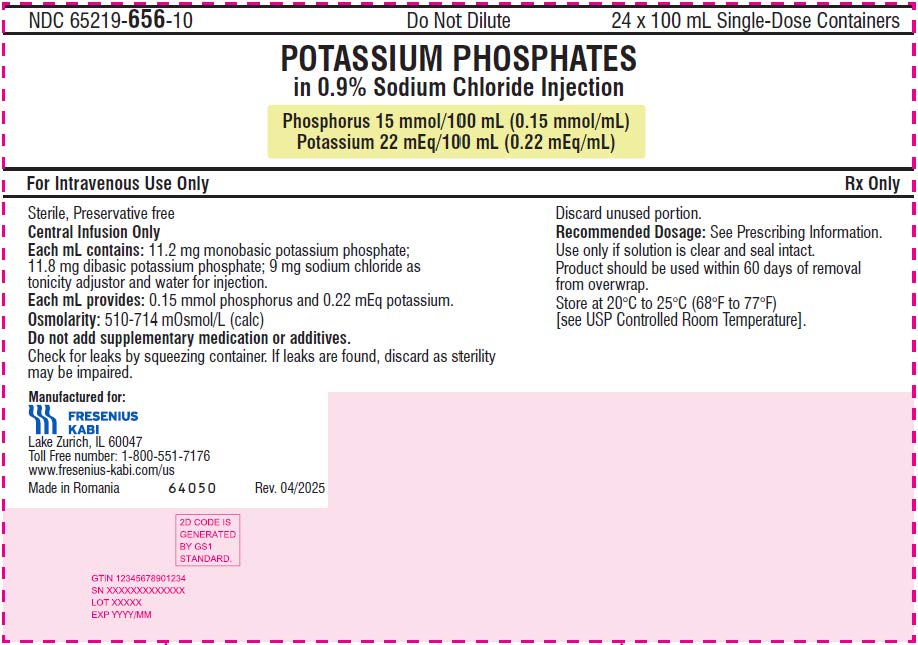
- The 100 mL ready-to-use container of this Potassium Phosphates in Sodium Chloride Injection product contains phosphorus 15 mmol (phosphorus 0.15 mmol/mL) and potassium 22 mEq (potassium 0.22 mEq/mL) and is for intravenous infusion into a central vein.
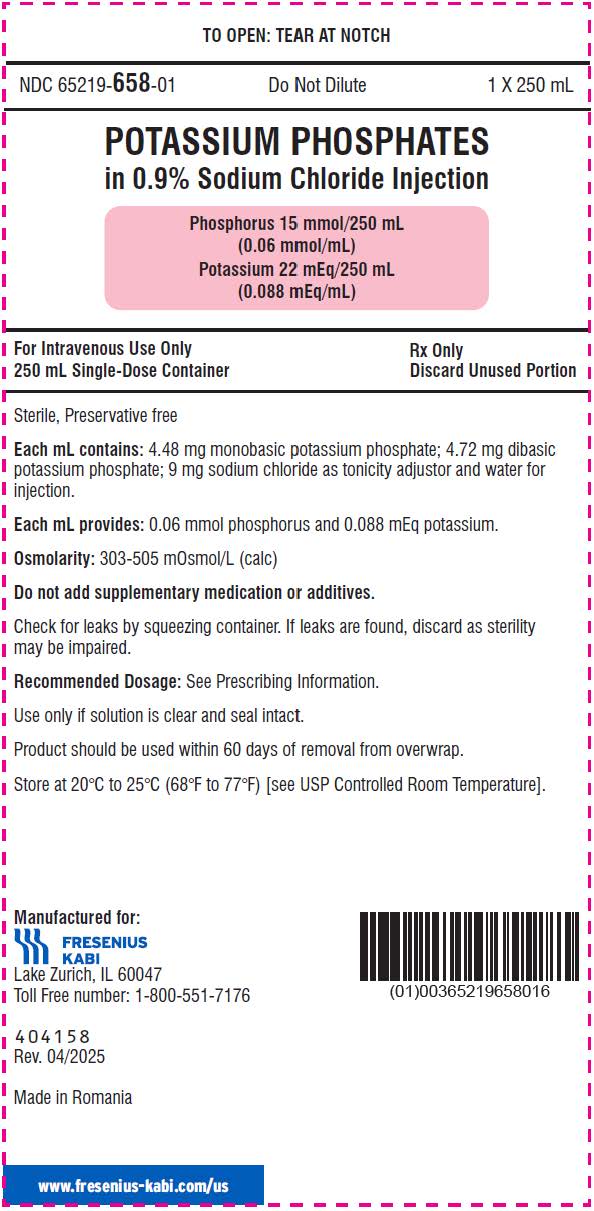
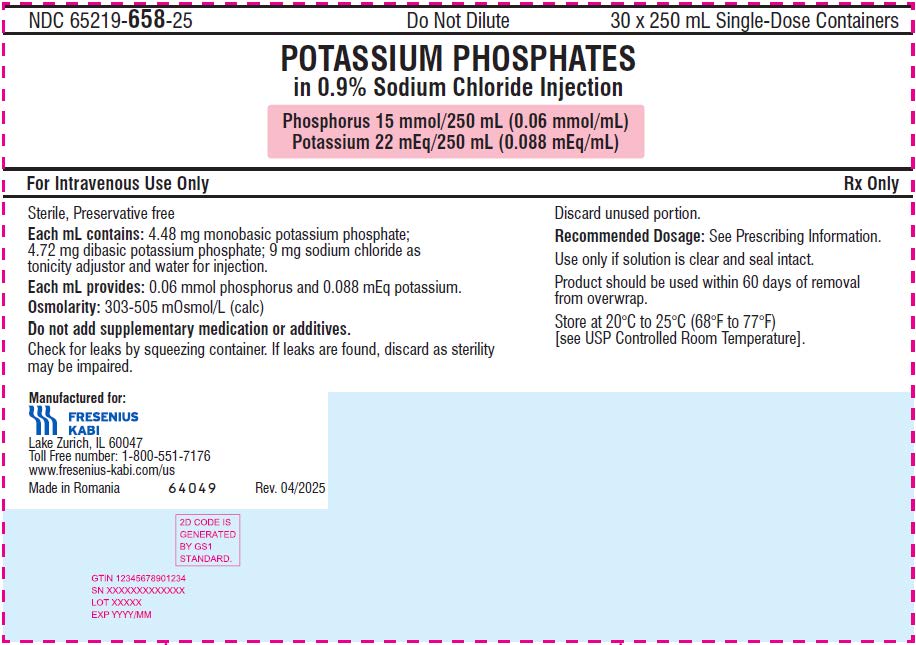
- The 250 mL ready-to-use container of this Potassium Phosphates in Sodium Chloride Injection product contains phosphorus 15 mmol and potassium 22 mEq (phosphorus 0.06 mmol/mL and potassium 0.088 mEq/mL) and is for intravenous infusion into a central OR peripheral vein.
2.3 Recommended Dosage
- The phosphorus doses in Table 1 are general recommendations for an initial or single dose of potassium phosphates and are intended for most patients who weigh 40 kg or greater. Based upon clinical requirements, some patients may require a lower or higher dose.
- The maximum initial or single dose of phosphorus is 45 mmol (potassium 66 mEq) [see Warnings and Precautions (5.1)].
- Consider overall volume status of the patient when determining whether Potassium Phosphates in Sodium Chloride Injection is an appropriate product for phosphorus replacement.
- Monitor serum phosphorus, potassium, calcium, and magnesium concentrations.
Table 1: Recommended Initial or Single Dose of Potassium Phosphates in Sodium Chloride injection to Correct Hypophosphatemia in Adults and Pediatric Patients Weighing 40 kg or Greater a Serum phosphorus reported using 2.5 mg/dL as the lower end of the reference range for healthy adults. Serum phosphorus concentrations may vary depending on the assay used and the laboratory reference range.
b Weight is in terms of actual body weight. Limited information is available regarding dosing of patients significantly above ideal body weight; consider using an adjusted body weight for these patients.
c This single-dose preparation of Potassium Phosphates in Sodium Chloride Injection contains phosphorus 15 mmol and potassium 22 mEq. Additional dose(s) following the initial dose may be needed in some patients.Serum Phosphorus Concentrationa
Phosphorus Dosageb, c
Corresponding Potassium Content
1.8 mg/dL to lower end of the reference rangea
0.16 mmol/kg to 0.31 mmol/kg
0.23 mEq/kg to 0.46 mEq/kg
1 mg/dL to 1.7 mg/dL
0.32 mmol/kg to 0.43 mmol/kg
0.47 mEq/kg to 0.63 mEq/kg
Less than 1 mg/dL
0.44 mmol/kg to 0.64 mmol/kgc
0.64 mEq/kg to 0.94 mEq/kg
Concentration and Intravenous Infusion Rate
Selection of the ready-to-use container solution concentration and the infusion rate is dependent upon whether administration will be through a peripheral or central venous catheter. The infusion rate may also be adjusted based on patient characteristics and the specific institution policy.
The maximum recommended concentration and infusion rates for Potassium Phosphates in Sodium Chloride Injection are shown in Table 2 for adults and pediatric patients weighing 40 kg or greater.
Table 2: Maximum Recommended Concentration and Infusion Rate of Potassium Phosphates in Sodium Chloride Injection for Adults and Pediatric Patients Weighing 40 kg or Greater Route of Administration
Maximum Concentration
Maximum Infusion Rate
Peripheral Venous Catheter
Phosphorus 6.8 mmol/100 mL (potassium 10 mEq/100 mL)
Phosphorus 6.8 mmol/hour
(potassium 10 mEq/hour)
Central Venous Catheter
Phosphorus 18 mmol/100 mL (potassium 26.4 mEq/100 mL)
Phosphorus 15 mmol/hour
(potassium 22 mEq/hour)
Continuous electrocardiographic (ECG) monitoring and infusion through a central venous catheter is recommended for infusion rates higher than potassium 10 mEq/hour.
Repeated Dosing
Additional dose(s) following the initial dose may be needed in some patients. Prior to administration of additional doses, assess the patient clinically, obtain serum phosphorus, calcium, and potassium concentrations and adjust the dose accordingly.
2.4 Recommended Dosage in Patients with Renal Impairment
- In patients with moderate renal impairment (eGFR ≥30 mL/min/1.73 m2 to <60 mL/min/1.73 m2), start at the low end of the dose range [see Dosage and Administration (2.3), Use in Specific Populations (8.6)].
- In patients with severe renal impairment (eGFR less than 30 mL/min/1.73m2), Potassium Phosphates in Sodium Chloride Injection is contraindicated [see Contraindications (4)].
-
3 DOSAGE FORMS AND STRENGTHS
Injection:
- phosphorus 15 mmol/100 mL (0.15 mmol/mL) and potassium 22 mEq/100 mL (0.22 mEq/mL) in a clear, colorless solution in a ready-to-use, single-dose container.
- phosphorus 15 mmol/250 mL (0.06 mmol/mL) and potassium 22 mEq/250 mL (0.088 mEq/mL) in a clear, colorless solution in a ready-to-use, single-dose container.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Cardiac Adverse Reactions with Bolus or Rapid Intravenous Administration
Intravenous administration of potassium phosphates to correct hypophosphatemia in single-doses of phosphorus 50 mmol and greater and/or at rapid infusion rates (over 1 to 3 hours) has resulted in death, cardiac arrest, cardiac arrhythmia (including QT prolongation), hyperkalemia, hyperphosphatemia, and seizures [see Overdosage (10)].
Continuous electrocardiographic (ECG) monitoring is recommended for infusion rates higher than potassium 10 mEq/hour [see Dosage and Administration (2.1, 2.2)].
5.2 Hyperkalemia
Potassium Phosphates in Sodium Chloride Injection may increase the risk of hyperkalemia, including life-threatening cardiac events, especially when administered in excessive doses or by rapid intravenous infusion [see Warnings and Precautions (5.1)]. Patients with renal impairment and are at increased risk of developing life-threatening hyperkalemia, when administered intravenous potassium. Potassium Phosphates in Sodium Chloride Injection is contraindicated in patients with severe renal impairment (eGFR less than 30 mL/min/1.73 m2) and dosage adjustment is required for patients with moderate renal impairment (eGFR ≥30 mL/min/1.73 m2 to <60 mL/min/1.73 m2) [see Dosage and Administration (2.2), Contraindications (4), Use in Specific Populations (8.6)].
Other patients at increased risk of hyperkalemia include those with severe adrenal insufficiency or treated concurrently with other drugs that cause or increase the risk of hyperkalemia [see Drug Interactions (7.1)]. Patients with cardiac disease may be more susceptible to the adverse effects of hyperkalemia.
Consider the amount of potassium from all sources when determining the dose of Potassium Phosphates in Sodium Chloride Injection and do not exceed the maximum age-appropriate recommended daily amount of potassium.
When administering Potassium Phosphates in Sodium Chloride Injection to correct hypophosphatemia, check the serum potassium concentration prior to administration [see Dosage and Administration (2.2)].
Continuous electrocardiographic (ECG) monitoring is recommended for infusion rates higher than 10 mEq/hour [see Warnings and Precautions (5.1), Dosage and Administration (2.3)].
5.3 Pulmonary Embolism due to Pulmonary Vascular Precipitates
Pulmonary vascular emboli and pulmonary distress related to precipitates in the pulmonary vasculature have been described in patients receiving admixed products containing calcium and phosphate or parenteral nutrition. The cause of precipitate formation has not been determined in all cases; however, in some fatal cases, pulmonary emboli occurred as a result of calcium phosphate precipitates. Precipitation has occurred following passage through an in-line filter; in vivo precipitate formation may also have occurred. If signs of pulmonary distress occur, stop the infusion and initiate a medical evaluation. In addition to inspection of the solution, the infusion set and catheter should also be checked periodically for precipitates. [see Dosage and Administration (2.1)].
5.4 Hyperphosphatemia and Hypocalcemia
Hyperphosphatemia can occur with intravenous administration of potassium phosphates, especially in patients with renal impairment [see Contraindications (4)]. Hyperphosphatemia can cause the formation of insoluble calcium phosphorus products with consequent hypocalcemia, neurological irritability with tetany, nephrocalcinosis with acute kidney injury, and more rarely, cardiac irritability with arrhythmias.
Obtain serum calcium concentrations prior to administration and normalize the calcium before administering Potassium Phosphates in Sodium Chloride Injection. Potassium Phosphates in Sodium Chloride Injection is contraindicated in patients with hyperphosphatemia and/or hypercalcemia [see Contraindications (4)].
Monitor serum phosphorus and calcium concentrations during treatment with Potassium Phosphates in Sodium Chloride Injection [see Dosage and Administration (2.3)].
5.5 Hypomagnesemia
Intravenous infusion of phosphate has been reported to cause a decrease in serum magnesium (and calcium) concentrations when administered to patients with hypercalcemia and diabetic ketoacidosis. Monitor serum magnesium concentrations during treatment.
5.6 Vein Damage and Thrombosis
The infusion of hypertonic solutions into a peripheral vein may result in vein irritation, vein damage, and/or thrombosis. The primary complication of peripheral administration is venous thrombophlebitis, which manifests as pain, erythema, tenderness or a palpable cord. Remove the catheter as soon as possible and initiate appropriate medical treatment if thrombophlebitis develops.
When administered peripherally to correct hypophosphatemia, a generally recommended maximum concentration is phosphorus 6.8 mmol/100 mL (potassium 10 mEq/100 mL) [see Dosage and Administration (2.2)].
5.7 Laboratory Monitoring
Monitor serum phosphorus, potassium, calcium, and magnesium concentrations during treatment [see Dosage and Administration (2.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Serious Cardiac Adverse Reactions with Bolus or Rapid Intravenous Administration [see Warnings and Precautions (5.1)]
- Hyperkalemia [see Warnings and Precautions (5.2)]
- Pulmonary Embolism due to Pulmonary Vascular Precipitates [see Warnings and Precautions (5.3)]
- Hyperphosphatemia and Hypocalcemia [see Warnings and Precautions (5.4)]
- Hypomagnesemia [see Warnings and Precautions (5.5)]
- Vein Damage and Thrombosis [see Warnings and Precautions (5.6)]
The following adverse reactions have been reported in clinical studies or post-marketing reports in patients receiving intravenously administered potassium phosphates. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Metabolism and Nutrition Disorders: hyperkalemia, hyperphosphatemia, hypocalcemia, hypovolemia, osmotic diuresis, pulmonary embolism
Cardiac Disorders: arrhythmia, bradycardia, cardiac arrest, chest pain, ECG changes, edema, heart block, hypotension
Respiratory, Thoracic, and Mediastinal Disorders: dyspnea
Renal and Urinary Disorders: acute phosphate nephropathy (i.e., nephrocalcinosis with acute kidney injury), decreased urine output, transition to chronic kidney disease
Gastrointestinal Disorders: diarrhea, stomach pain
Musculoskeletal and Connective Tissue Disorders: weakness
Nervous System Disorders: confusion, lethargy, paralysis, paresthesia
-
7 DRUG INTERACTIONS
7.1 Other Products that Increase Serum Potassium
Administration of Potassium Phosphates in Sodium Chloride Injection to patients treated concurrently or recently with products that increase serum potassium (e.g., potassium-sparing diuretics, ACE inhibitors, angiotensin II receptor antagonists, digoxin, or the immunosuppressants tacrolimus and cyclosporine) increases the risk of severe and potentially fatal hyperkalemia, especially in the presence of other risk factors for hyperkalemia [see Warnings and Precautions (5.2)]. Avoid use of Potassium Phosphates in Sodium Chloride Injection in patients receiving such products. If use cannot be avoided, closely monitor serum potassium concentrations [see Dosage and Administration (2.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Administration of the recommended dose of Potassium Phosphates in Sodium Chloride Injection is not expected to cause major birth defects, miscarriage, or adverse maternal or fetal outcomes. Consider intravenous potassium phosphate replacement if correction of hypophosphatemia via the enteral route is not possible (see Clinical Considerations). Animal reproduction studies have not been conducted with Potassium Phosphates in Sodium Chloride Injection.The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated Maternal and/or Embryo-Fetal Risk
Phosphorus is an essential mineral element. Intravenous replacement with potassium phosphate should be considered if a pregnant woman requires intravenous replacement to correct hypophosphatemia when the enteral route is not possible, insufficient, or contraindicated.
8.2 Lactation
Risk Summary
Phosphorus and potassium are present in human milk. Administration of the recommended dose of Potassium Phosphates in Sodium Chloride Injection is not expected to cause harm to a breastfed infant. There is no information on the effects of potassium phosphates on milk production. The development and health benefits of breastfeeding should be considered along with the mother's clinical need for Potassium Phosphates in Sodium Chloride Injection and any potential adverse effects on the breastfed child from Potassium Phosphates in Sodium Chloride Injection or from the underlying maternal condition.8.4 Pediatric Use
Safety and effectiveness of Potassium Phosphates in Sodium Chloride Injection have been established in pediatric patients weighing 40 kg or more as a source of phosphorus to correct hypophosphatemia when oral or enteral replacement is not possible, insufficient, or contraindicated. This Potassium Phosphates in Sodium Chloride Injection product is not approved for use in pediatric patients who weigh less than 40 kg because they would require only a fraction of the ready-to-use container [see Dosage and Administration (2.1)].
8.5 Geriatric Use
In general, dose selection of Potassium Phosphates in Sodium Chloride Injection for an elderly patient should be cautious, starting at the low end of the dosing range because of the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. It may be useful to monitor renal function during treatment [see Use in Specific Populations (8.6)].
8.6 Renal Impairment
Potassium and phosphorus are known to be substantially excreted by the kidney and the risk of adverse reactions to Potassium Phosphates in Sodium Chloride Injection may be greater in patients with impaired renal function [see Warnings and Precautions (5.2, 5.4)].
Potassium Phosphates in Sodium Chloride Injection is contraindicated in patients with severe renal impairment (eGFR less than 30 mL/min/1.73 m2) due to the risk of hyperkalemia [see Contraindications (4)].
In patients with moderate renal impairment (eGFR ≥ 30 mL/min/1.73 m2 to < 60 mL/min/1.73 m2), start at the low end of the dosage range and monitor serum potassium, phosphorus, calcium, and magnesium concentrations [see Dosage and Administration (2.4)].
-
10 OVERDOSAGE
Hyperphosphatemia
Administration of excessive doses of intravenous potassium phosphates as a single dose ranging from approximately 50 to 270 mmol phosphorus and/or at rapid infusion rates (over 1 to 3 hours) has resulted in death, cardiac arrest, cardiac arrhythmia (including QT prolongation), hyperkalemia, hyperphosphatemia, seizures, and tetany.
Hyperphosphatemia is particularly a risk in patients with renal failure.
Hyperphosphatemia leads in turn to hypocalcemia, which may be severe, and to ectopic calcification, particularly in patients with initial hypercalcemia. Tissue calcification may cause hypotension and organ damage and result in acute renal failure.
Hyperkalemia
Excessive administration of phosphates given as potassium salts may also cause hyperkalemia. Manifestations of hyperkalemia include:
- Disturbances in cardiac conduction and arrhythmias, including bradycardia, heart block, asystole, ventricular tachycardia, ventricular fibrillation, and death.
- Hypotension.
- Muscle weakness including paresthesia, muscular and respiratory paralysis.
Management
In the event of overdosage, discontinue infusions containing potassium phosphates immediately and institute general supportive measures, including ECG monitoring, laboratory monitoring, and correction of serum electrolyte concentrations, especially potassium, phosphorus, calcium, and magnesium.
-
11 DESCRIPTION
Potassium Phosphates in Sodium Chloride Injection is a phosphorus replacement product.
It is a sterile, non-pyrogenic, solution containing a mixture of monobasic potassium phosphate and dibasic potassium phosphate in sodium chloride. It is supplied as a 100 mL and 250 mL ready-to-use single dose container for intravenous use and does not require further dilution.
Monobasic Potassium Phosphate is chemically designated KH2PO4, molecular weight 136.09, white, odorless crystals or granules freely soluble in water.
Dibasic Potassium Phosphate is chemically designated K2HPO4, molecular weight 174.18, colorless or white granular salt freely soluble in water.
For 100 mL single-dose container:
Each mL contains 11.2 mg of monobasic potassium phosphate and 11.8 mg of dibasic potassium phosphate. Each mL contains 0.15 mmol phosphorus (equivalent to 4.65 mg phosphorus), and 0.22 mEq potassium (equivalent to 8.50 mg of potassium).
For 250 mL single-dose container:
Each mL contains 4.48 mg of monobasic potassium phosphate and 4.72 mg of dibasic potassium phosphate. Each mL contains 0.06 mmol phosphorus (equivalent to 1.86 mg phosphorus), and 0.088 mEq potassium (equivalent to 3.40 mg of potassium).
Note: 1 mmol of phosphorus is equal to 1 mmol phosphate. In addition, each mL of solution contains 9 mg sodium chloride for isotonicity. The pH is 6.0 to 7.0.
This product contains no more than 100 mcg/L of aluminum.
The osmolality is 500-700 mOsmol/kg for the 100 mL single-dose container and 300-500 mOsmol/kg for the 250 mL single-dose container.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Phosphorus in the form of organic and inorganic phosphate has a variety of biochemical functions in all organs and tissues, including critical roles in nucleic acid structure, energy storage and transfer, cell signaling, cell membrane composition and structure, acid-base balance, mineral homeostasis, and bone mineralization.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of potassium phosphates have not been fully characterized.
12.3 Pharmacokinetics
Distribution
Approximately 85% of serum phosphates is free and ultra-filterable and 15% is protein-bound.
Elimination
Intravenously infused phosphates not taken up by the tissues are excreted almost entirely in the urine. Serum phosphorus is believed to be filterable by the renal glomeruli, and the major portion of filtered phosphorus (greater than 80%) is actively reabsorbed by the tubules.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Potassium Phosphates in Sodium Chloride Injection is a clear, colorless solution supplied as:
Unit of Sale
Strength
Each Unit
NDC: 65219-656-10
Box containing 24 units
Phosphorus 15 mmol/100 mL (0.15 mmol/mL) and Potassium 22mEq/100 mL (0.22 mEq/mL)
NDC: 65219-656-01
100 mL single-dose container
NDC: 65219-658-25
Box containing 30 units
Phosphorus 15 mmol/250 mL (0.06 mmol/mL) and Potassium 22mEq/250 mL (0.088 mEq/mL)
NDC: 65219-658-01
250 mL single-dose container
The container closure is not made with natural rubber latex. Non-PVC, Non-DEHP, Sterile.
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature]. Product should be used within 60 days of removal from overwrap.
Each ready-to-use infusion container contains no preservatives. Do NOT dilute prior to use. Discard any unused portion in the single-dose container immediately.
-
17 PATIENT COUNSELING INFORMATION
Inform patients, caregivers, or home healthcare providers of the following risks of Potassium Phosphates in Sodium Chloride Injection:
- Advise patients of the serious cardiac risks (e.g., death, cardiac arrest, cardiac arrhythmia, hyperkalemia, hyperphosphatemia, and seizures) associated with rapid administration of Potassium Phosphate in Sodium Chloride Injection [see Warnings and Precautions (5.1)].
- Advise patients that Potassium Phosphate in Sodium Chloride Injection may increase the risk of hyperkalemia when administered in excessive doses or by rapid intravenous infusion [see Warnings and Precautions (5.2)].
- Advise patients that hyperphosphatemia can occur, especially in patients with renal impairment, which can result in hypocalcemia [see Warnings and Precautions (5.4)].
- Advise patients that Potassium Phosphate in Sodium Chloride Injection has been reported to cause hypomagnesemia when administered to patients with hypercalcemia and diabetic ketoacidosis [see Warnings and Precautions (5.5)].
Manufactured For:
Lake Zurich, IL 60047
Toll free number: 1-800-551-7176
www.fresenius-kabi.com/us
451846
- PRINCIPAL DISPLAY PANEL – 100 mL Container
- PRINCIPAL DISPLAY PANEL – 100 mL Overwrap
- PRINCIPAL DISPLAY PANEL – 100 mL Carton LBL
- PRINCIPAL DISPLAY PANEL – 250 mL Container
- PRINCIPAL DISPLAY PANEL – 250 mL Overwrap
- PRINCIPAL DISPLAY PANEL – 250 mL Carton LBL
-
INGREDIENTS AND APPEARANCE
POTASSIUM PHOSPHATES
potassium phosphates injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65219-656 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MONOBASIC POTASSIUM PHOSPHATE (UNII: 4J9FJ0HL51) (POTASSIUM CATION - UNII:295O53K152, PHOSPHATE ION - UNII:NK08V8K8HR) MONOBASIC POTASSIUM PHOSPHATE 11.2 mg in 1 mL DIBASIC POTASSIUM PHOSPHATE (UNII: CI71S98N1Z) (POTASSIUM CATION - UNII:295O53K152, PHOSPHATE ION - UNII:NK08V8K8HR) DIBASIC POTASSIUM PHOSPHATE 11.8 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 9 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65219-656-10 24 in 1 CARTON 06/15/2025 1 NDC: 65219-656-01 1 in 1 POUCH 1 100 mL in 1 BAG; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212832 06/15/2025 POTASSIUM PHOSPHATES
potassium phosphates injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65219-658 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MONOBASIC POTASSIUM PHOSPHATE (UNII: 4J9FJ0HL51) (POTASSIUM CATION - UNII:295O53K152, PHOSPHATE ION - UNII:NK08V8K8HR) MONOBASIC POTASSIUM PHOSPHATE 4.48 mg in 1 mL DIBASIC POTASSIUM PHOSPHATE (UNII: CI71S98N1Z) (POTASSIUM CATION - UNII:295O53K152, PHOSPHATE ION - UNII:NK08V8K8HR) DIBASIC POTASSIUM PHOSPHATE 4.72 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 9 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65219-658-25 30 in 1 CARTON 06/15/2025 1 NDC: 65219-658-01 1 in 1 POUCH 1 250 mL in 1 BAG; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212832 06/15/2025 Labeler - Fresenius Kabi USA, LLC (013547657)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.