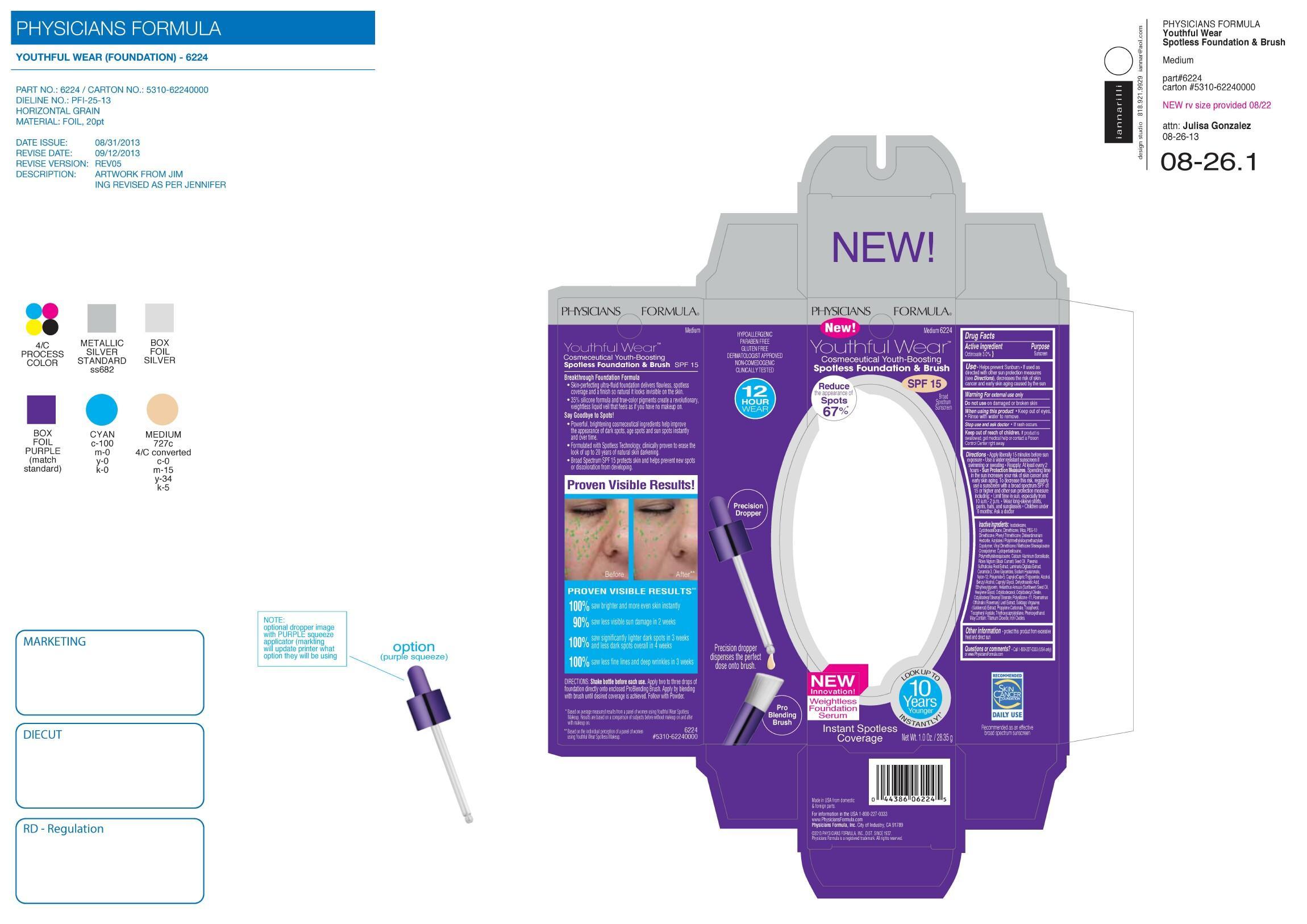
Youthful Wear Spotless Foundation by Physicians Formula Inc YW Spotless Fdtn-PF
Youthful Wear Spotless Foundation by
Drug Labeling and Warnings
Youthful Wear Spotless Foundation by is a Otc medication manufactured, distributed, or labeled by Physicians Formula Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
YOUTHFUL WEAR SPOTLESS FOUNDATION SPF 15- octinoxate lotion
Physicians Formula Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
YW Spotless Fdtn-PF
| YOUTHFUL WEAR SPOTLESS FOUNDATION
SPF 15
octinoxate lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Physicians Formula Inc (021261805) |
| Registrant - Physicians Formula Inc (021261805) |
Revised: 12/2019
Document Id: 9a0471bd-5421-3519-e053-2995a90abf9f
Set id: 35aa2853-55ef-4dd8-929f-453066ab6f8b
Version: 4
Effective Time: 20191218
Physicians Formula Inc