HEATHER- norethindrone tablet
HEATHER by
Drug Labeling and Warnings
HEATHER by is a Prescription medication manufactured, distributed, or labeled by Glenmark Pharmaceuticals Inc., USA, Glenmark Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each pale yellow HEATHER tablet provides a continuous oral contraceptive regimen of 0.35 mg norethindrone USP daily, and the inactive ingredients include corn starch, lactose monohydrate, magnesium stearate, povidone, talc, D&C Yellow No. 10 aluminum lake and FD&C Yellow No. 6 aluminium lake.
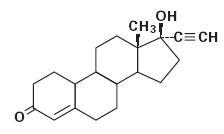
The chemical name for norethindrone USP is 17-Hydroxy-19-Nor-17α-pregn-4-en-20-yn-3-one. The structural formula follows:
Norethindrone USP
Therapeutic class = oral contraceptive
-
CLINICAL PHARMACOLOGY
1. Mode of Action
HEATHER progestin-only oral contraceptives prevent conception by suppressing ovulation in approximately half of users, thickening the cervical mucus to inhibit sperm penetration, lowering the mid-cycle LH and FSH peaks, slowing the movement of the ovum through the fallopian tubes, and altering the endometrium.
2. Pharmacokinetics
Absorption:
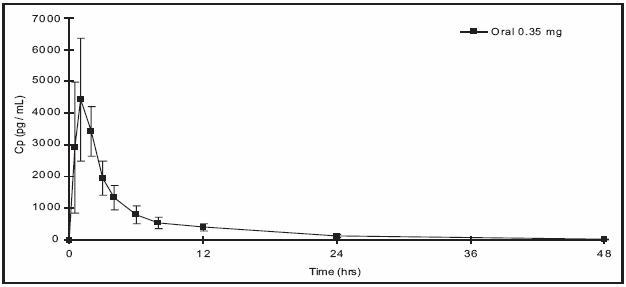
Norethindrone is rapidly absorbed with maximum plasma concentrations occurring within 1 to 2 hours after HEATHER administration (see Table 1). Norethindrone appears to be completely absorbed following oral administration; however, it is subject to first pass metabolism resulting in an absolute bioavailability of approximately 65%.
Peak plasma concentrations occur approximately 1 hour after administration (mean Tmax 1.2 hours). The mean (SD) Cmax was 4816.8 (1532.6) pg/mL and generally occurred within 1 hour (mean) of tablet administration, ranging from 0.5 to 2 hours. The mean (SD) Cavg was 885 (250) pg/mL, however, the mean concentration at 24 hrs was 130 (47) pg/mL.
Table 1 provides summary statistics of the pharmacokinetic parameters associated with single dose HEATHER administration.
Table 1: Mean ± SD Pharmacokinetic Parameters Following Single Dose Administration of HEATHER in 12 Healthy Female Subjects Under Fasting Conditions Pharmacokinetic Parameter
Norethindrone 0.35 mg
Tmax (hr)
1.2 ± 0.5
Cmax (pg/mL)
4817 ± 1533
AUC(0-48) (pgh/mL)
21233 ± 6002
t1/2 (h)
7.7 ± 0.5
The food effect on the rate and extent of norethindrone absorption after HEATHER administration has not been evaluated.
Distribution:
Following oral administration, norethindrone is 36% bound to sex hormone-binding globulin (SHBG) and 61% bound to albumin. Volume of distribution of norethindrone is approximately 4 L/kg.
Metabolism:
Norethindrone undergoes extensive biotransformation, primarily via reduction, followed by sulfate and glucuronide conjugation; less than 5% of a norethindrone dose is excreted unchanged; greater than 50% and 20-40% of a dose is excreted in urine and feces, respectively. The majority of metabolites in the circulation are sulfate, with glucuronides accounting for most of the urinary metabolites.
Excretion:
Plasma clearance rate for norethindrone has been estimated to be approximately 600 L/day. Norethindrone is excreted in both urine and feces, primarily as metabolites. The mean terminal elimination half-life of norethindrone following single dose administration of HEATHER is approximately 8 hours.
-
INDICATIONS AND USAGE
2. Efficacy
If used perfectly, the first-year failure rate for progestin-only oral contraceptives is 0.5%. However, the typical failure rate is estimated to be closer to 5%, due to late or omitted pills. The following table lists the pregnancy rates for users of all major methods of contraception.
Table 2. Percentage of Women Experiencing an Unintended Pregnancy During the First Year of Typical Use and the First Year of Perfect Use of Contraception and the Percentage Continuing Use at the End of the First Year. United States. Emergency Contraceptive Pill: Treatment initiated within 72 hours after unprotected intercourse reduces the risk of pregnancy by at least 75%.*
Lactational Amenorrhea Method: LAM is a highly effective, temporary method of contraception.†
Source: Trussell J, Contraceptive Efficacy. In: Hatcher RA, Trussell J, Stewart F, Cates W, Stewart GK, Kowal D, Guest F, Contraceptive Technology: Seventeenth Revised Edition. New York, NY: Irvington Publishers, 1998.- * The treatment schedule is one dose within 72 hours after unprotected intercourse, and a second dose 12 hours after the first dose. The Food and Drug Administration has declared the following brands of oral contraceptives to be safe and effective for emergency contraception: Ovral® (1 dose is 2 white pills), Alesse® (1 dose is 5 pink pills), Nordette® or Levlen® (1 dose is 4 yellow pills).
- † However, to maintain effective protection against pregnancy, another method of contraception must be used as soon as menstruation resumes, the frequency or duration of breastfeeds is reduced, bottle feeds are introduced, or the baby reaches 6 months of age
- ‡ Among couples attempting to avoid pregnancy, the percentage who continue to use a method for one year
- § Among typical couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any reason.
- ¶ Among couples who initiate use of a method (not necessarily for the first time), and who use it perfectly (both consistently and correctly), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason
- # The percentage of women becoming pregnant noted in columns (2) and (3) are based on data from populations where contraception is not used and from women who cease using contraception in order to become pregnant. Among such populations, about 89% become pregnant within one year. This estimate was lowered slightly (to 85%) to represent the percentage that would become pregnant within one year among women now relying on reversible methods of contraception if they abandoned contraception altogether
- Þ Foams, creams, gels, vaginal suppositories, and vaginal film
- ß Cervical mucus (ovulation) method supplemented by calendar in the pre-ovulatory and basal body temperature in the post-ovulatory phases
- à With spermicidal cream or jelly
- è Without spermicides
% of Women Experiencing an Unintended Pregnancy within the First Year of Use
% of Women Continuing Use at One Year‡
Method
(1)
Typical Use§
(2)
Perfect Use¶
(3)
(4)
Chance#
85
85
SpermicidesÞ
26
6
40
Periodic abstinence
25
63
Calendar
9
Ovulation Method
3
Sympto-Thermalß
2
Post-Ovulation
1
Capà
Parous Women
40
26
42
Nulliparous Women
20
9
56
Sponge
Parous Women
40
20
42
Nulliparous Women
20
9
56
Diaphragmà
20
6
56
Withdrawal
19
4
Condomè
Female (Reality)
21
5
56
Male
14
3
61
Pill
5
71
Progestin only
0.5
Combined
0.1
IUDs
Progesterone T
2.0
1.5
81
Copper T 380A
0.8
0.6
78
LNg 20
0.1
0.1
81
Depo-Provera®
0.3
0.3
70
Levonorgestrel Implants (Norplant®)
0.05
0.05
88
Female Sterilization
0.5
0.5
100
Male Sterilization
0.15
0.10
100
-
CONTRAINDICATIONS
Progestin-only oral contraceptives (POPs) should not be used by women who currently have the following conditions:
- Known or suspected pregnancy
- Known or suspected carcinoma of the breast.
- Undiagnosed abnormal genital bleeding
- Hypersensitivity to any component of this product
- Benign or malignant liver tumors
- Acute liver disease.
-
WARNINGS
Cigarette smoking greatly increases the possibility of suffering heart attacks and strokes. Women who use oral contraceptives are strongly advised not to smoke.
HEATHER does not contain estrogen and, therefore, this insert does not discuss the serious health risks that have been associated with the estrogen component of combined oral contraceptives. The health care provider is referred to the prescribing information of combined oral contraceptives for a discussion of those risks, including, but not limited to, an increased risk of serious cardiovascular disease in women who smoke, carcinoma of the breast and reproductive organs, hepatic neoplasia, and changes in carbohydrate and lipid metabolism. The relationship between progestin-only oral contraceptives and these risks have not been established and there are no studies definitely linking progestin-only pill (POP) use to an increased risk of heart attack or stroke.
The physician should remain alert to the earliest manifestation of symptoms of any serious disease and discontinue oral contraceptive therapy when appropriate.
1. Ectopic pregnancy
The incidence of ectopic pregnancies for progestin-only oral contraceptive users is 5 per 1000 woman-years. Up to 10% of pregnancies reported in clinical studies of progestin-only oral contraceptive users are extrauterine. Although symptoms of ectopic pregnancy should be watched for, a history of ectopic pregnancy need not be considered a contraindication to use of this contraceptive method. Health providers should be alert to the possibility of an ectopic pregnancy in women who become pregnant or complain of lower abdominal pain while on progestin-only oral contraceptives.
2. Delayed follicular atresia/Ovarian cysts
If follicular development occurs, atresia of the follicle is sometimes delayed, and the follicle may continue to grow beyond the size it would attain in a normal cycle. Generally these enlarged follicles disappear spontaneously. Often they are asymptomatic; in some cases they are associated with mild abdominal pain. Rarely they may twist or rupture, requiring surgical intervention.
3. Irregular genital bleeding
Irregular menstrual patterns are common among women using progestin-only oral contraceptives. If genital bleeding is suggestive of infection, malignancy or other abnormal conditions, such nonpharmacologic causes should be ruled out. If prolonged amenorrhea occurs, the possibility of pregnancy should be evaluated.
4. Carcinoma of the breast and reproductive organs
Some epidemiologic studies of oral contraceptive users have reported an increased relative risk of developing breast cancer, particularly at a younger age and apparently related to duration of use. These studies have predominantly involved combined oral contraceptives and there is insufficient data to determine whether the use of POPs similarly increase the risk. Women with breast cancer should not use oral contraceptives because the role of female hormone in breast cancer has not been fully determined.
Some studies suggest that oral contraceptive use has been associated with an increase in the risk of cervical intraepithelial neoplasia in some populations of women. However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors. There is insufficient data to determine whether the use of POPs increases the risk of developing cervical intraepithelial neoplasia.
5. Hepatic neoplasia.
Benign hepatic adenomas are associated with combined oral contraceptive use, although the incidence of benign tumors is rare in the United States. Rupture of benign, hepatic adenomas may cause death through intra-abdominal hemorrhage.
Studies from Britain and the U.S. have shown as increased risk of developing hepatocellular carcinoma in combined oral contraceptive users. However, these cancers are rare. There is insufficient data to determine whether POPs increase the risk of developing hepatic neoplasia.
-
PRECAUTIONS
1. General
Patients should be counseled that oral contraceptives do not protect against transmission of HIV (AIDS) and other sexually transmitted diseases (STDs) such as Chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B, and syphilis.
2. Physical examination and follow-up
It is considered good medical practice for sexually active women using oral contraceptives to have annual history and physical examinations. The physical examination may be deferred until after initiation of oral contraceptives if requested by the woman and judged appropriate by the clinician.
3. Carbohydrate and lipid metabolism
Some users may experience slight deterioration in glucose tolerance, with increases in plasma insulin, but women with diabetes mellitus who use progestin-only oral contraceptives do not generally experience changes in their insulin requirements. Nonetheless, prediabetic and diabetic women in particular should be carefully monitored while taking POPs.
Lipid metabolism is occasionally affected in that HDL, HDL2, and apolipoprotein A-I and A-II may be decreased; hepatic lipase may be increased. There is no effect on total cholesterol, HDL3, LDL, or VLDL.
4. Drug Interactions
Change in contraceptive effectiveness associated with co-administration of other products:
a. Anti-infective agents and anticonvulsants
Contraceptive effectiveness may be reduced when hormonal contraceptives are co-administered with antibiotics, anticonvulsants, and other drugs that increase the metabolism of contraceptive steroids. This could result in unintended pregnancy or breakthrough bleeding. Examples include rifampin, barbiturates, phenylbutazone, phenytoin, carbamazepine, felbamate, oxcarbazepine, topiramate, and griseofulvin.
b. Anti-HIV protease inhibitors
Several of the anti-HIV protease inhibitors have been studied with co-administration of oral contraceptives; significant changes (increase and decrease) in the plasma levels of the estrogen and progestin have been noted in some cases. The safety and efficacy of OC products may be affected with the co-administration of anti-HIV protease inhibitors. Health care providers should refer to the label of the individual anti-HIV protease inhibitors for further drug-drug interaction information.
5. Interactions with laboratory tests
The following endocrine tests may be affected by progestin-only oral contraceptive use
- Sex hormone-binding globulin (SHBG) concentrations may be decreased.
- Thyroxine concentrations may be decreased, due to a decrease in thyroid binding globulin (TBG).
7. Pregnancy
Many studies have found no effects on fetal development associated with long-term use of contraceptive doses of oral progestins. The few studies of infant growth and development that have been conducted have not demonstrated significant adverse effects. It is nonetheless prudent to rule out suspected pregnancy before initiating any hormonal contraceptive use.
8. Nursing mothers
Small amounts of progestin pass into the breast milk, resulting in steroid levels in infant plasma of 1-6% of the levels of maternal plasma. However, isolated post-market cases of decreased milk production have been reported in POPs. Very rarely, adverse effects in the infant/child have been reported, including jaundice.
9. Fertility following discontinuation
The limited available data indicate a rapid return of normal ovulation and fertility following discontinuation of progestin-only oral contraceptives.
10. Headache/Migraine
If you have a headache or a worsening migraine headache with a new pattern that is recurrent, persistent, or severe, this requires discontinuation of oral contraceptives and evaluation of the cause.
11. Gastrointestinal
Diarrhea and/or vomiting may reduce hormone absorption resulting in decreased serum concentrations.
12. Pediatric use
Safety and efficacy of HEATHER has been established in women of reproductive age. Safety and efficacy are expected to be the same for postpubertal adolescents under the age of 16 and for users 16 years and older. Use of this product before menarche is not indicated.
Information for the Patient
-
- See PATIENT LABELING for detailed information.
- Counseling issues.
The following points should be discussed with prospective users before prescribing progestin-only oral contraceptives
- The necessity of taking pills at the same time every day, including throughout all bleeding episodes.
- The need to use a backup method such as condoms and spermicides for the next 48 hours whenever a progestin-only oral contraceptive is taken 3 or more hours late
- The potential side effects of progestin-only oral contraceptives, particularly menstrual irregularities
- The need to inform the clinician of prolonged episodes of bleeding, amenorrhea or severe abdominal pain
- The importance of using a barrier method in addition to progestin-only oral contraceptives if a woman is at risk of contracting or transmitting STDs/HIV
-
ADVERSE REACTIONS
- Menstrual irregularity is the most frequently reported side effect
- Frequent and irregular bleeding are common, while long duration of bleeding episodes and amenorrhea are less likely.
- Headache, breast tenderness, nausea, and dizziness are increased among progestin-only oral contraceptive users in some studies
- Androgenic side effects such as acne, hirsutism, and weight gain occur rarely.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
To achieve maximum contraceptive effectiveness, HEATHER must be taken exactly as directed. One tablet is taken every day, at the same time. Administration is continuous, with no interruption between pill packs. See PATIENT LABELING for detailed instructions.
-
HOW SUPPLIED
HEATHER (Norethindrone tablets USP, 0.35 mg) are available in 28-tablet dispensers as pale yellow round, flat faced beveled edged, uncoated tablets with ‘303’ debossed on one side and ‘G’ on the other side (NDC: 68462-303-29).
- STORAGE
- SPL UNCLASSIFIED SECTION
-
DETAILED INFORMATION FOR THE PATIENT
Patients should be counseled that oral contraceptives do not protect against transmission of HIV (AIDS) and other sexually transmitted diseases (STDs) such as Chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B, and syphilis.
INTRODUCTION
This leaflet is about birth control pills that contain one hormone, a progestin. Please read this leaflet before you begin to take your pills. It is meant to be used along with talking with your doctor or clinic.
Progestin-only pills are often called “POPs” or “the minipill”. POPs have less progestin than the combined birth control pill (or “the pill”) which contains both an estrogen and a progestin.
HOW EFFECTIVE ARE POPS?
About 1 in 200 (0.5%) POPs users will get pregnant in the first year if they all take POPs perfectly (that is, on time, every day). About 1 in 20 (5%) “typical” POPs users (including women who are late taking pills or miss pills) gets pregnant in the first year of use. The following table will help you compare the efficacy of different methods.
IUD: 1-2%
Depo-Provera® (injectable progesterone): 0.3%
Norplant® System (levonorgestrel implants): 0.1%
Diaphragm with spermicides: 18%
Spermicides alone: 21%
Male condom alone: 12%
Female condom alone: 21%
Cervical cap:
Women who have never given birth : 18%
Women who have given birth : 36%
Periodic abstinence: 20%
No methods: 85%
HOW DO POPS WORK?
- They make the cervical mucus at the entrance to the womb (the uterus) too thick for the sperm to get through to the egg.
- They prevent ovulation (release of the egg from the ovary) in about half the time
- They also affect other hormones, the fallopian tubes and the lining of the uterus.
YOU SHOULD NOT TAKE POPS
- If there is any chance you may be pregnant.
- If you have breast cancer.
- If you have bleeding between your periods which has not been diagnosed.
- If you are taking certain drugs for epilepsy (seizures) or for TB. (See USING POPS WITH OTHER MEDICINES below).
- If you are hypersensitive or allergic to any component of this product.
- If you have liver tumors, either benign or cancerous.
- If you have acute liver disease.
RISKS OF TAKING POPS
WARNING: If you have sudden or severe pain in your lower abdomen or stomach area, you may have an ectopic pregnancy or an ovarian cyst. If this happens, you should contact your doctor or clinic immediately.
- Ectopic pregnancy. An ectopic pregnancy is a pregnancy outside the womb. Because POPs protect against pregnancy, the chance of having a pregnancy outside the womb is very low. If you do get pregnant while taking POPs, you have slightly higher chance that the pregnancy will be ectopic than do users of some other birth control methods.
- Ovarian cysts. These cysts are small sacs of fluid in the ovary. They are more common among POP users than among users of most other birth control methods. They usually disappear without treatment and rarely cause problems.
-
Cancer of the reproductive organs and breasts. Some studies in women who use combined oral contraceptives that contain both estrogen and a progestin have reported an increase in the risk of developing breast cancer, particularly at a younger age and apparently related to duration of use. There is insufficient data to determine whether the use of POPs similarly increases this risk.
Some studies have found an increase in the incidence of cancer of the cervix in women who use oral contraceptives. However, this finding may be related to factors other than the use of oral contraceptives and there is insufficient data to determine whether the use of POPs increases the risk of developing cancer of the cervix. - Liver tumors. In rare cases, combined oral contraceptives can cause benign but dangerous liver tumors. These benign liver tumors can rupture and cause fatal internal bleeding. In addition, a possible but not definite association has been found with combined oral contraceptives and liver cancers in studies in which a few women who developed these very rare cancers were found to have used combined oral contraceptives for long periods of time. There is insufficient data to determine whether POPs increase the risk of liver tumors.
SEXUALLY TRANSMITTED DISEASES (STDS)
WARNING: POPs do not protect against getting or giving someone HIV (AIDS) or any other STD, such as Chlamydia, gonorrhea, genital warts or herpes.
SIDE EFFECTS
- Irregular bleeding. The most common side effect of POPs is a change in menstrual bleeding. Your periods may be either early or late, and you may have some spotting between periods. Taking pills late or missing pills can also result in some spotting or bleeding.
- Other side effects. Less common side effects include headaches, tender breasts, nausea and dizziness. Weight gain, acne and extra hair on your face and body have been reported, but are rare.
If you are concerned about any of these side effects, check with your doctor or clinic.
USING POPS WITH OTHER MEDICINES
Before taking a POP, inform your health care provider of any other medication, including over-the-counter medicine that you may be taking.
If you are taking medicines for seizures (epilepsy) or tuberculosis (TB), tell your doctor or clinic. These medicines can make POPs less effective:
Medicines for seizures:
- Phenytoin (Dilantin®)
- Carbamazepine (Tegretol®)
- Phenobarbital
Medicine for TB:
- Rifampin (Rifampicin)
Before you begin any new medicines be sure your doctor or clinic knows you are taking birth control pills that contain a progestin.
HOW TO TAKE POPS
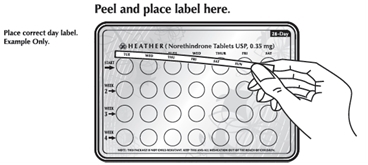
1. Pick the day label strip that starts with the first day of your period.
2. Place this day label strip on the tablet blister card over the area that has the days of the week (starting with Sunday) imprinted in the plastic.
Note: If the first day of your period is a Sunday, you can skip steps #1 and #2.
IMPORTANT POINTS TO REMEMBER
- POPs must be taken at the same time every day, so choose a time and then take the pill at the same time every day. Every time you take a pill late, and especially if you miss a pill, you are more likely to get pregnant.
- Start the next pack the day after the last pack is finished. There is no break between packs. Always have your next pack of pills ready.
- You may have some menstrual spotting between periods. Do not stop taking your pills if this happens.
- If you vomit soon after taking a pill, use a backup method (such as condom and/or spermicide) for 48 hours.
- If you want to stop taking POPs, you can do so at any time, but, if you remain sexually active and don’t wish to become pregnant, be certain to use another birth control method.
- If you are not sure about how to take POPs, ask your doctor or clinic.
STARTING POPS
- It’s best to take your first POP on the first day of your menstrual period.
- If you decide to take your first POP on another day, use a backup method (such as a condom and/or spermicide) every time you have sex during the next 48 hours.
- If you have had a miscarriage or an abortion, you can start POPs the next day.
IF YOU ARE LATE OR MISS TAKING YOUR POPS
-
If you are more than 3 hours late or you miss one or more POPs:
- 1. TAKE a missed pill as soon as you remember that you missed it,
- 2. THEN go back to taking POPs at your regular time.
- 3. BUT be sure to use a backup method (such as condom and/or spermicide) every time you have sex for the next 48 hours.
- If you are not sure what to do about the pills you have missed, keep taking POPs and use a backup method until you can talk to your doctor or clinic.
IF YOU ARE BREASTFEEDING
- If you are fully breastfeeding (not giving your baby any food or formula), you may start your pills 6 weeks after delivery.
- If you are partially breastfeeding (giving your baby some food or formula), you should start taking pills by 3 weeks after delivery.
IF YOU ARE SWITCHING PILLS
- If you are switching from the combined pills to POPs, take the first POP the day after you finish the last active combined pill. Do not take any of the 7 inactive pills from the combined pill pack. You should know that many women have irregular periods after switching to POPs, but this is normal and to be expected.
- If you are switching from POPs to the combined pills, take the first active combined pill on the first day of your period, even if your POPs pack is not finished.
- If you switch to another brand of POPs, start the new brand anytime.
- If you are breastfeeding, you can switch to another method of birth control at any time, except do not switch to the combined pills until you stop breastfeeding or at least until 6 months after delivery.
PREGNANCY WHILE ON THE PILL
If you become pregnant, or think you might be, stop taking POPs and contact your physician. Even though research has shown that POPs do not cause harm to the unborn baby, it is always best not to take any drugs or medicines that you don’t need when you are pregnant.
You should get a pregnancy test:
- If your period is late and you took one or more pills late or missed taking them and had sex without a backup method.
- Anytime you miss 2 periods in a row.
WILL POPS AFFECT YOUR ABILITY TO GET PREGNANT LATER?
If you want to become pregnant, simply stop taking POPs. POPs will not delay your ability to get pregnant.
BREASTFEEDING
If you are breastfeeding, POPs will not affect the quality or amount of your breast milk or the health of your nursing baby. However, isolated cases of decreased milk production have been reported. If you suspect that you are not producing enough milk for your baby, contact your doctor or clinic.
OVERDOSE
No serious problems have been reported when many pills were taken by accident, or even by a small child, so there is usually no reason to treat an overdose.
OTHER QUESTIONS OR CONCERNS
Cigarette smoking greatly increases the possibility of suffering heart attacks and strokes. Women who use oral contraceptives are strongly advised not to smoke.
Diabetic women taking POPs do not generally require changes in the amount of insulin they are taking. However, your physician may monitor you more closely under these conditions.
If you have any questions or concerns, check with your doctor or clinic. You can also ask for the more detailed “professional package labeling” written for doctors and other health care providers.
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEATHER
norethindrone tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68462-303 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NORETHINDRONE (UNII: T18F433X4S) (NORETHINDRONE - UNII:T18F433X4S) NORETHINDRONE 0.35 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TALC (UNII: 7SEV7J4R1U) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color YELLOW Score no score Shape ROUND Size 7mm Flavor Imprint Code G;303 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68462-303-29 3 in 1 CARTON 04/23/2010 1 28 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090454 04/23/2010 Labeler - Glenmark Pharmaceuticals Inc., USA (130597813) Establishment Name Address ID/FEI Business Operations Glenmark Pharmaceuticals Limited 677318665 ANALYSIS(68462-303) , MANUFACTURE(68462-303)
Trademark Results [HEATHER]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HEATHER 98018168 not registered Live/Pending |
Butter Pat Industries, LLC 2023-05-30 |
 HEATHER 86532383 4812424 Live/Registered |
Heather, LLC 2015-02-11 |
 HEATHER 78311957 not registered Dead/Abandoned |
Heather Design Limited 2003-10-10 |
 HEATHER 77726233 3901324 Live/Registered |
GLENMARK PHARMACEUTICALS INC., USA 2009-04-30 |
 HEATHER 74070032 1644357 Dead/Cancelled |
Heather Classics, Inc. 1990-06-18 |
 HEATHER 73823743 1694917 Dead/Cancelled |
HEATHER, EUNICE PAMELA 1989-09-05 |
 HEATHER 73537568 1368996 Dead/Cancelled |
TROJAN LUGGAGE COMPANY, THE 1985-05-14 |
 HEATHER 73483467 not registered Dead/Abandoned |
DIET INSTITUTE, INC., THE 1984-06-04 |
 HEATHER 73422209 1290019 Dead/Cancelled |
Clarke Checks, Inc. 1983-04-18 |
 HEATHER 73125833 1095137 Dead/Expired |
PEPPERITE THERMOGRAPHERS, INC. 1977-05-09 |
 HEATHER 73077462 1048503 Dead/Cancelled |
American Greetings Corporation 0000-00-00 |
 HEATHER 72312263 0871713 Dead/Expired |
MATTEL, INC. 1968-11-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.