Infants Ibuprofen Concentrated Drops,Ibuprofen Oral Suspension Drug Facts
Ibuprofen by
Drug Labeling and Warnings
Ibuprofen by is a Otc medication manufactured, distributed, or labeled by Strides Pharma Inc, Strides Pharma Global Pte. Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
IBUPROFEN - ibuprofen suspension
Strides Pharma Inc
----------
Infants Ibuprofen Concentrated Drops,
Ibuprofen Oral Suspension
Drug Facts
Uses
- reduces fever
- relieves minor aches and pains due to the common cold, flu, headache and toothaches
Warnings
Allergy alert
Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if your child:
- has had stomach ulcers or bleeding problems
- takes a blood thinning (anticoagulant) or steroid drug
- takes other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- takes more or for a longer time than directed
Heart attack and stroke warning
NSAIDs, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
Do not use
- if the child has ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
Ask a doctor before use if
- stomach bleeding warning applies to your child
- child has problems or serious side effects from taking pain relievers or fever reducers
- child has a history of stomach problems, such as heartburn
- child has high blood pressure, heart disease, liver cirrhosis, kidney disease, or had a stroke
- child has not been drinking fluids
- child has lost a lot of fluid due to vomiting or diarrhea
- child is taking a diuretic
Ask a doctor or pharmacist before use if the child is
- under a doctor's care for any serious condition
- taking any other drug
Stop use and ask a doctor if
- child experiences any of the following signs of stomach bleeding:
- feels faint
- vomits blood
- has bloody or black stools
- has stomach pain that does not get better
- child has symptoms of heart problems or stroke:
- chest pain
- trouble breathing
- weakness in one part or side of body
- slurred speech
- leg swelling
- child does not get any relief within first day (24 hours) of treatment
- fever or pain gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
OTC - KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed
- shake well before using
- find right dose on chart. If possible, use weight to dose; otherwise use age.
- repeat dose every 6-8 hours, if needed
- do not use more than 4 times a day
- measure with the dosing device provided. Do not use with any other device.
| Weight (lb)
| Age (mos)
| Dose (mL)
|
| under 6 mos | ask a doctor |
|
| 12-17 lb | 6-11 mos | 1.25 mL |
| 18-23 lb | 12-23 mos | 1.875 mL |
Other Information
- one dose lasts 6-8 hours
- store at 20-25°C (68-77°F)
- see bottom of box for lot number and expiration date
Inactive ingredients
citric acid monohydrate, edetate disodium dihydrate, glycerin, microcrystalline cellulose, natural and artificial flavors, polysorbate 80, propylene glycol, purified water, sodium benzoate, sodium carboxymethylcellulose, sorbitol solution, sucrose and xanthan gum
Questions or comments?
Call Strides Pharma Inc. weekdays from 9 AM to 5 PM EST at 1-877-244-9825 or ask your pharmacist, doctor or health care professional or visit www.strides.com.
Manufactured by:
Strides Pharma Science Limited
Bengaluru - 562106, India.
Distributed by:
Strides Pharma Inc.,
East Brunswick, NJ 08816
Revised: 05/2022
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
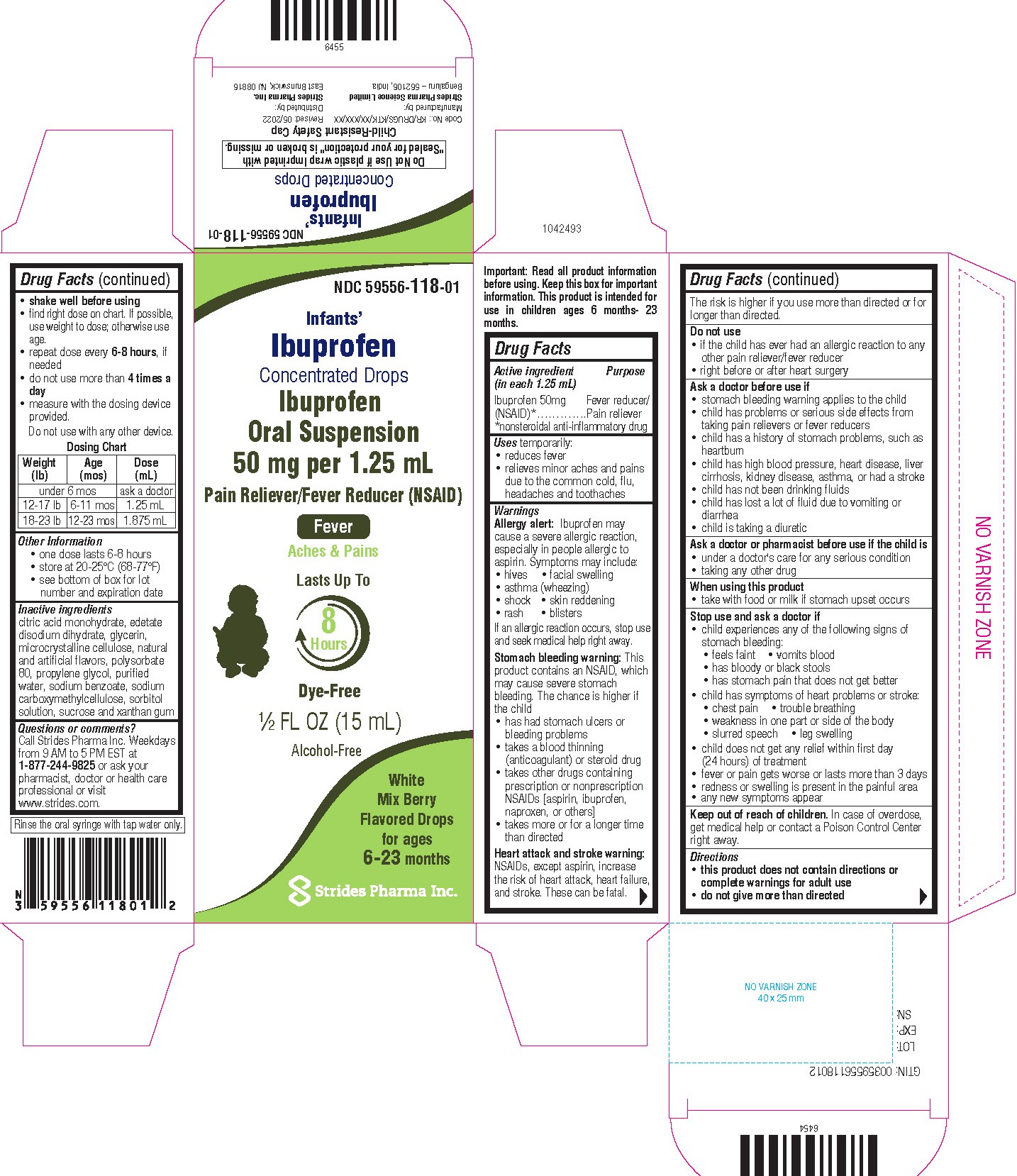
NDC: 59556-149-01
For Ages 6-23 Months
Ibuprofen Concentrated Drops
Oral Suspension 50 mg per 5 mL
Pain Reliever / Fever Reducer (NSAID)
Lasts up to
8 hours
Alcohol Free
Mix Berry Flavor
1/2 fl oz (15 mL)
NDC: 59556-149-02
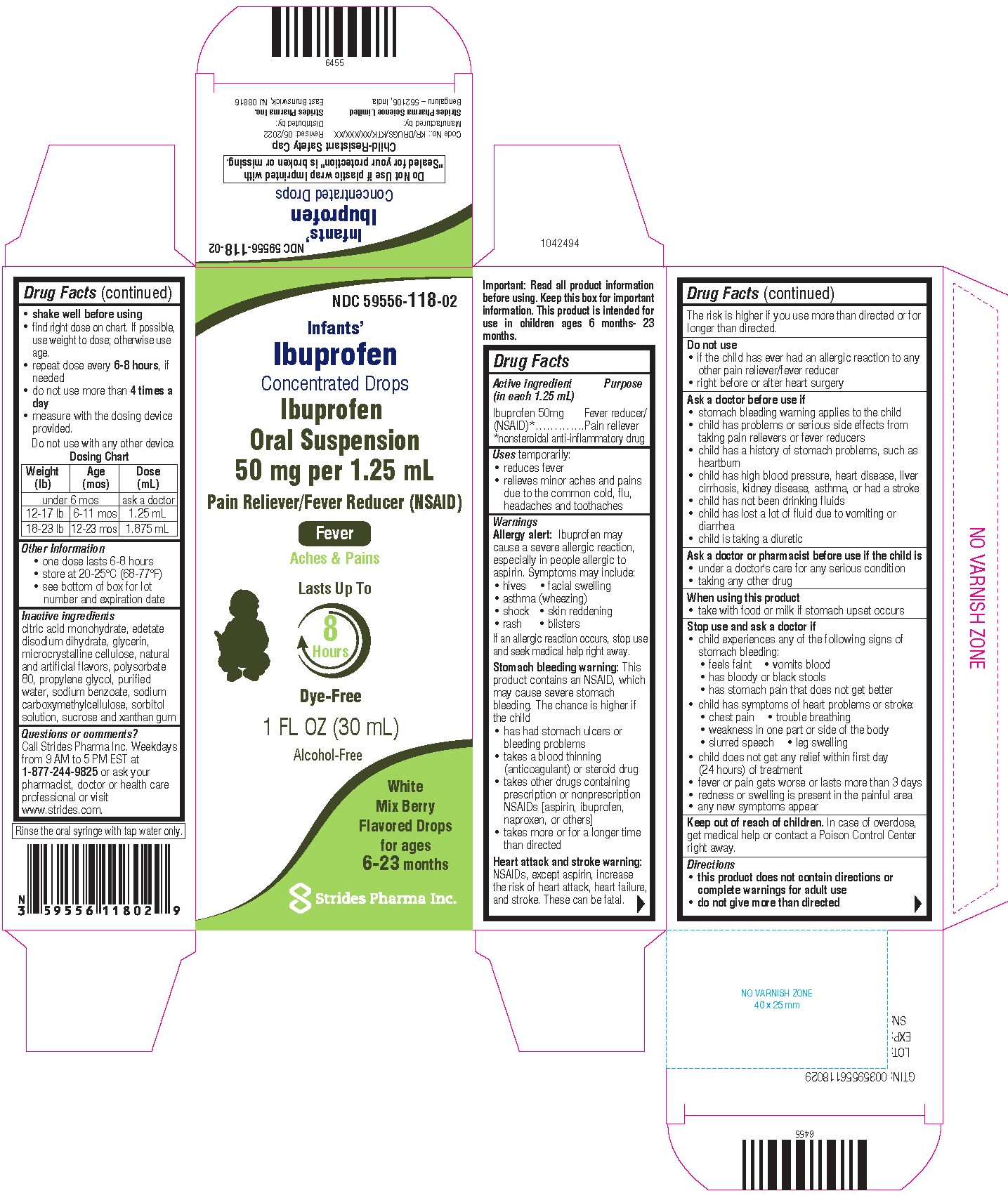
For Ages 6-23 Months
Ibuprofen Concentrated Drops
Oral Suspension 50 mg per 5 mL
Pain Reliever / Fever Reducer (NSAID)
Lasts up to
8 hours
Alcohol Free
Mix Berry Flavor
1 fl oz (30 mL)
| IBUPROFEN
ibuprofen suspension |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Strides Pharma Inc (078868278) |
| Registrant - Strides Pharma Global Pte. Ltd. (659220961) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.