EYEWASH STATION ADDITIVE CONCENTRATE- chlorhexidine gluconate and propylene glycol liquid
Eyewash Station Additive Concentrate by
Drug Labeling and Warnings
Eyewash Station Additive Concentrate by is a Otc medication manufactured, distributed, or labeled by Niagara Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Ingredients
- Purpose
- Use
- Warnings
-
Directions
- wear protective eyewear and gloves
- clean potable eyewash station and rinse with potable water
- partially fill station with potable water
- remove tamper evident seal and cap of bottle
- add entire contents of the bottle to the eyewash station container
- fill the station to the manufacturer's required level
- date and initial inspection tag
- station should be cleaned and refilled every 120 days when using this product
- in advance of emergency, add the concentrate to potable water to have a solution available
- Other information
- Questions ?
- SPL UNCLASSIFIED SECTION
-
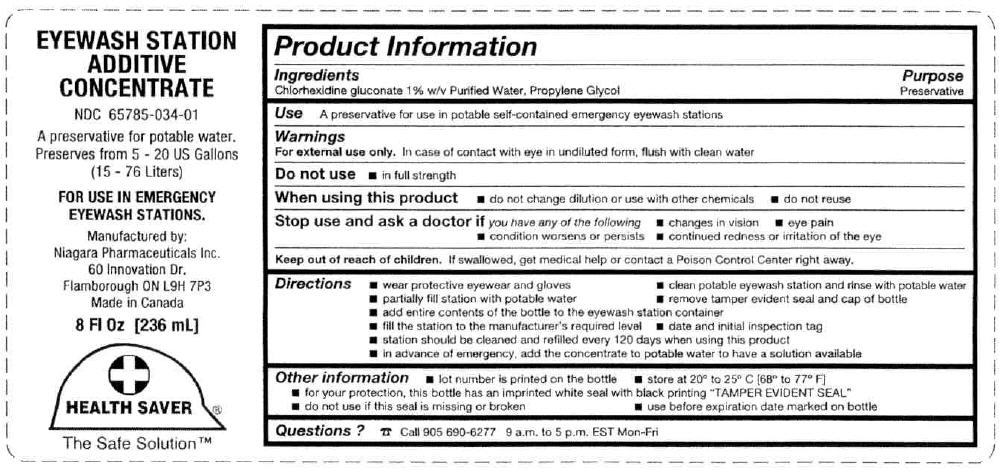
PRINCIPAL DISPLAY PANEL - 236 mL Bottle Label
EYEWASH STATION
ADDITIVE
CONCENTRATENDC: 65785-034-01
A preservative for potable water.
Preserves from 5 - 20 US Gallons
(15 - 76 Liters)FOR USE IN EMERGENCY
EYEWASH STATIONS.Manufactured by:
Niagara Pharmaceuticals Inc.
60 Innovation Dr.
Flamborough ON L9H 7P3
Made in Canada8 Fl Oz [236 mL]
HEALTH SAVER®
The Safe Solution™
-
INGREDIENTS AND APPEARANCE
EYEWASH STATION ADDITIVE CONCENTRATE
chlorhexidine gluconate and propylene glycol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 65785-034 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 145.6 kg in 2800 L PROPYLENE GLYCOL (UNII: 6DC9Q167V3) (PROPYLENE GLYCOL - UNII:6DC9Q167V3) PROPYLENE GLYCOL 280 kg in 2800 L Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 2374.4 L in 2800 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65785-034-01 0.236 L in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 11/15/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part349 11/15/2011 Labeler - Niagara Pharmaceuticals Inc. (205477792) Registrant - Niagara Pharmaceuticals Inc. (205477792) Establishment Name Address ID/FEI Business Operations Niagara Pharmaceuticals Inc. 205477792 manufacture(65785-034)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.