Sennosides by Morepen Laboratories Limited SENNOSIDES tablet
Sennosides by
Drug Labeling and Warnings
Sennosides by is a Otc medication manufactured, distributed, or labeled by Morepen Laboratories Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient Section Label
- Purpose Section Label
- Indications & Usage Section Label
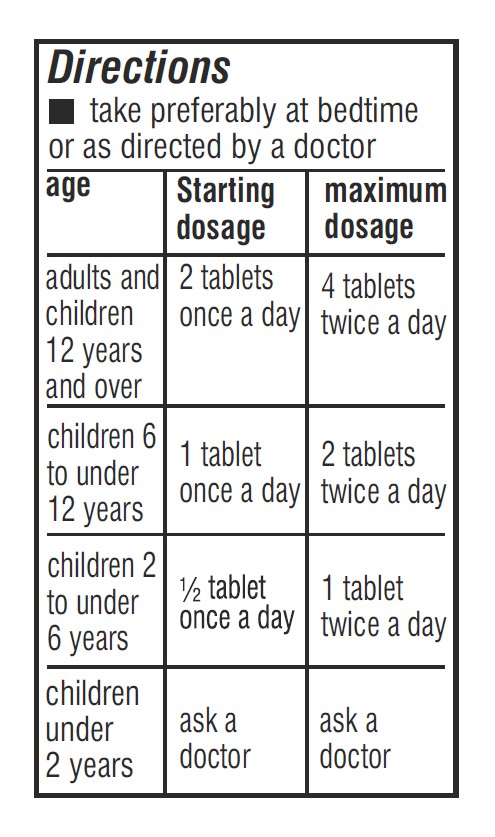
- Dosage & Administration Section Label
- Inactive Ingredients Section Label
- Questions Section Label
- Warnings Section
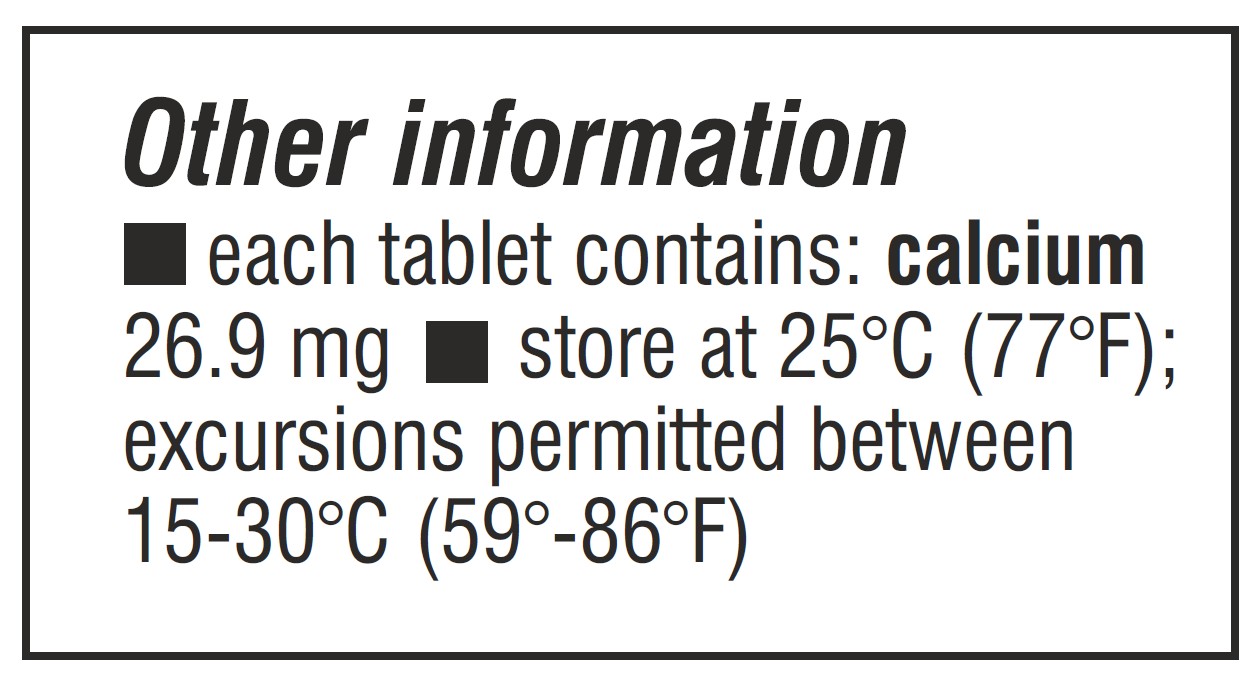
- STORAGE AND HANDLING
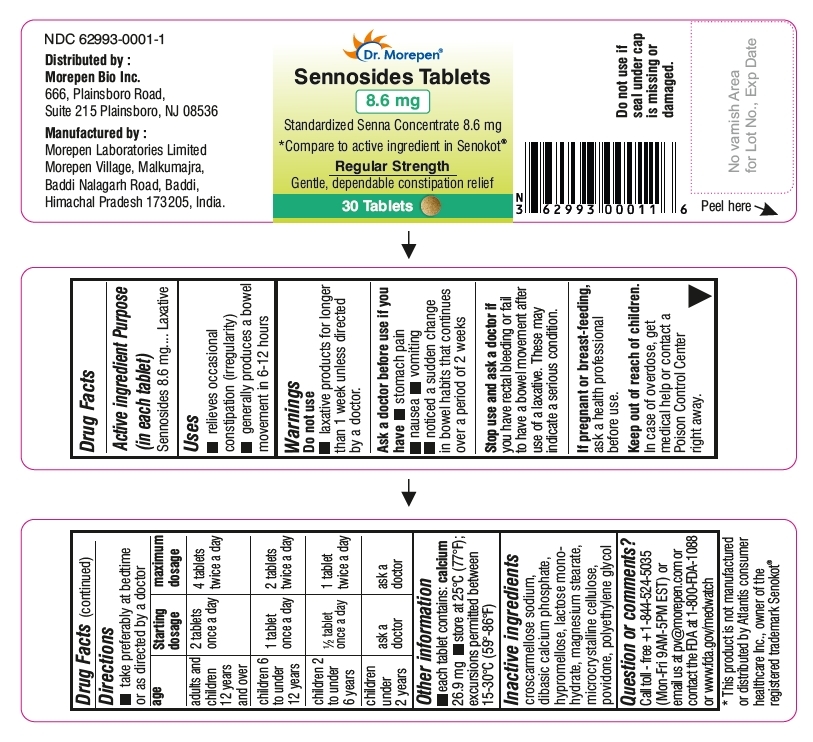
- 30 Tablets Bottle Label (NDC: 62993-0001-1)
- 100 Tablets Bottle Label (NDC: 62993-0001-2)
- 250 Tablets Bottle Label (NDC: 62993-0001-3)
- 1000 Tablets Bottle Label (NDC: 62993-0001-4)
- 3000 Tablets Bottle Label (NDC: 62993-0001-5)
- 30000 Tablets Drum Label (NDC: 62993-0001-6)
-
INGREDIENTS AND APPEARANCE
SENNOSIDES
sennosides tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62993-0001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) POVIDONE K30 (UNII: U725QWY32X) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Product Characteristics Color brown (Light grey to brown mottled) Score no score Shape ROUND Size 9mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62993-0001-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2025 2 NDC: 62993-0001-2 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2025 3 NDC: 62993-0001-3 250 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2025 4 NDC: 62993-0001-4 1000 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2025 5 NDC: 62993-0001-5 3000 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2025 6 NDC: 62993-0001-6 30000 in 1 DRUM; Type 0: Not a Combination Product 11/07/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 10/01/2025 Labeler - Morepen Laboratories Limited (650087067) Registrant - Morepen Laboratories Limited (772653251) Establishment Name Address ID/FEI Business Operations Morepen Laboratories Limited 772653251 manufacture(62993-0001) , analysis(62993-0001) , label(62993-0001) , pack(62993-0001)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.