Guaifenesin by KESIN PHARMA CORPORATION / Wittman Pharma, Inc. / Kesin Pharma GUAIFENESIN liquid
Guaifenesin by
Drug Labeling and Warnings
Guaifenesin by is a Otc medication manufactured, distributed, or labeled by KESIN PHARMA CORPORATION, Wittman Pharma, Inc., Kesin Pharma. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Guaifenesin Solution 5mL
- Description
- Inactive Ingredients
- Uses
-
Warnings
Ask a doctor before us if you have
cough that occurs with too much phlegm (mucus)
cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysemaStop and ask a doctor if
cough lasts mor than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
you are hypersensitive to any of the ingredients
If pregnant or breast-feeding, ask a health professional before use. -
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Professional Note: Guaifenesin has been shown to produce a color interference with certain clinical laboratory determinations of 5-hydroxyindoleacetic acid (5-HIAA) and vanillylmandelic acid (VMA).
-
Directions
Follow dosage below or use as directed by a physician.
Do not take more than 6 doses in any 24-hour periodAge Dose Adults and children 12 years and over 10 to 20mL (2 to 4 teaspoonfuls) every 4 hours Children 6 years to under 12 years 5 to 10mL (1 to 2 teaspoonfuls) every 4 hours Children 2 to under 6 years of age 2.5 to 5mL (1/2 to 1 teaspoonful) every 4 hours Children under 2 years of age Consult a physician -
How Supplied:
Guaifenesin Oral Solution is a clear, colorless solution with a grape flavor, free of visible foreign matter supplied in the following oral dosage forms:
NDC: 81033-102-05: 5mL unit dose cup
NDC: 81033-102-51: Case containing 100 unit dose cups of 5 mL packaged in 2 cartons of 50 unit dose cups each
NDC: 81033-102-10: 10mL unit dose cup
NDC: 81033-102-52: Case containing 100 unit dose cups of 10 mL packaged in 2 cartons of 50 unit dose cups each
NDC: 81033-102-15: 15 mL unit dose cup
NDC: 81033-102-53: Case containing 100 unit dose cups of 15 mL packaged in 2 cartons of 50 unit dose cups each - STORAGE
- QUESTIONS OR COMMENTS
-
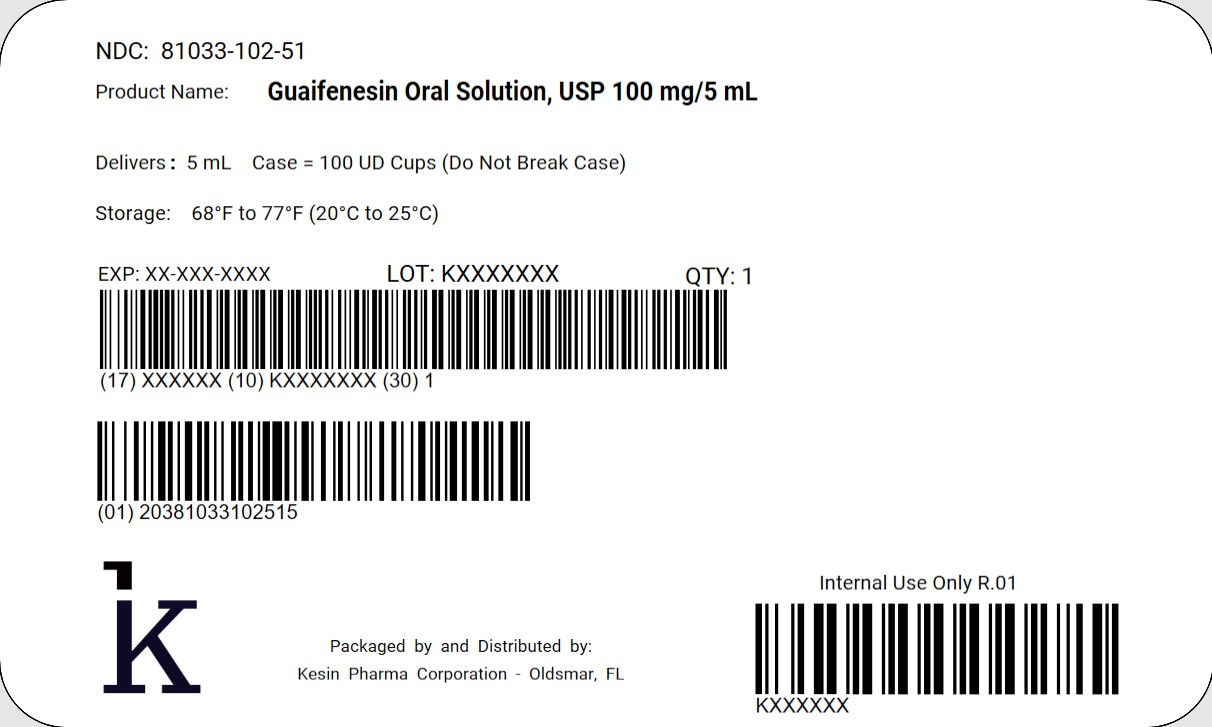
5 mL Unit Dose Cup Case Label
NDC: 81033-102-51
Guaifenesin Oral Solution, USP100 mg/5 mL
Delivers 5 mL
Case = 100 UD Cups (Do Not Break Case)
Store at 68°F to 77°F (20°C to 25°C)
QTY 1
-
10 mL Unit Dose Cup Case Label
NDC: 81033-102-52
Guaifenesin Oral Solution, USP 200 mg/10 mL
Delivers 10 mL
Case = 100 UD Cups (Do Not Break Case)
Store at 68°F to 77°F (20°C to 25°C)
QTY 1

-
15 mL Unit Dose Cup Case Label
NDC: 81033-102-53
Guaifenesin Oral Solution, USP 300 mg/15 mL
Delivers 15 mL
Case = 100 UD Cups (Do Not Break Case)
QTY 1

-
INGREDIENTS AND APPEARANCE
GUAIFENESIN
guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 81033-102 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) METHYLPARABEN (UNII: A2I8C7HI9T) AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) POTASSIUM CITRATE ANHYDROUS (UNII: 86R1NVR0HW) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 81033-102-51 50 in 1 CARTON 11/20/2023 1 NDC: 81033-102-05 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product 2 NDC: 81033-102-52 50 in 1 CARTON 11/20/2023 2 NDC: 81033-102-10 10 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/20/2023 Labeler - KESIN PHARMA CORPORATION (117447816) Establishment Name Address ID/FEI Business Operations Kesin Pharma 117447816 pack(81033-102) , label(81033-102) Establishment Name Address ID/FEI Business Operations Belleview Biosciences 131968803 manufacture(81033-102) , label(81033-102) , analysis(81033-102)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
