PENTETATE ZINC TRISODIUM injection, solution, concentrate
Pentetate zinc trisodium by
Drug Labeling and Warnings
Pentetate zinc trisodium by is a Prescription medication manufactured, distributed, or labeled by hameln pharma plus gmbh, Siegfried Hameln Gmbh. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PENTETATE ZINC TRISODIUM INJECTION safely and effectively.
See full prescribing information for PENTETATE ZINC TRISODIUM INJECTION.
Pentetate zinc trisodium injection (Zn-DTPA)
For intravenous or inhalation administration
Initial U.S. Approval: 2004WARNING: ASTHMA EXACERBATION WITH NEBULIZATION and DEPLETION OF TRACE METALS DURING THERAPY
See full prescribing information for complete boxed warning.
- Nebulized Zn-DTPA may be associated with asthma exacerbation. (5.1)
- Zn-DTPA is associated with depletion of trace metals. The risk for depletion increases when Zn-DTPA is administered over several months. Monitor serum zinc levels, serum creatinine, BUN, electrolytes, urinalysis and blood cell counts during Ca-DTPA or Zn-DTPA therapy. (2.4, 5.2)
INDICATIONS AND USAGE
Pentetate zinc trisodium injection is a radiomitigation chelator indicated for treatment of individuals with known or suspected internal contamination with plutonium, americium, or curium to increase the rates of elimination. (1)
DOSAGE AND ADMINISTRATION
Chelation treatment is most effective if administered within the first 24 hours. Administer Ca-DTPA, if available, as the initial dose. (2.1, 2.2)
If Ca-DTPA is not available during the first 24 hours,
- in adults and adolescents, administer intravenously a single 1.0 gram Zn-DTPA initial dose. (2.1)
- in children less than 12 years of age, administer intravenously a single 14 mg/kg Zn-DTPA initial dose, not to exceed 1.0 gram. (2.1)
After the first 24 hours, continue chelation therapy with Zn-DTPA:
- in adults and adolescents, administer intravenously 1.0 gram Zn-DTPA once daily. (2.1)
- in children less than 12 years of age, administer intravenously 14 mg/kg Zn-DTPA once daily, not to exceed 1.0 gram daily. (2.1)
See Full Prescribing Information for dose (2.1) and nebulized chelation therapy. (2.3)
DOSAGE FORMS AND STRENGTHS
1000 mg / 5 mL single-use ampoules. (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Nebulized chelation therapy may be associated with exacerbation of asthma. Monitor patients for signs and symptoms of asthma exacerbation when administering Zn-DTPA by the inhalation route. (5.1)
- Zn-DTPA is associated with depletion of endogenous trace metals (e.g., zinc, magnesium, manganese). (5.2)
- Take appropriate safety measures to minimize contamination of care-takers by contaminated body fluids. (5.3)
ADVERSE REACTIONS
There is limited experience with Zn-DTPA. Nebulized chelation therapy may be associated with exacerbation of asthma. Headache, light headedness, and pelvic pain have been reported. (6)
To report SUSPECTED ADVERSE REACTIONS, contact the hameln Pharmacovigilance Department at +44 (0) 7706 210 133 or drugsafety@hameln.co.uk or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Adequate and well-controlled drug-drug interaction studies in humans were not identified in the literature. (7)
USE IN SPECIFIC POPULATIONS
- Nursing Mothers: Women with known or suspected internal contamination with radiocontaminants should not breast feed, whether or not they are receiving chelation therapy. (8.3)
- Pediatric Use: Safety and effectiveness of intravenous Zn-DTPA were extrapolated from adults. Safety and effectiveness of nebulized route of administration have not been established. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ASTHMA EXACERBATION WITH NEBULIZATION and DEPLETION OF TRACE METALS DURING THERAPY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 General
2.3 Methods of Administration
2.4 Monitoring
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Asthma Exacerbation
5.2 Depletion of Body Trace Mineral Stores
5.3 Risks to Care-takers
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing mothers
8.4 Pediatric use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED
16.2 Storage
16.3 Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ASTHMA EXACERBATION WITH NEBULIZATION and DEPLETION OF TRACE METALS DURING THERAPY
- Nebulized Zn-DTPA may be associated with asthma exacerbation. (5.1)
- Zn-DTPA is associated with depletion of trace metals. The risk for depletion increases when Zn-DTPA is administered over several months. Monitor serum zinc levels, serum creatinine, BUN, electrolytes, urinalysis and blood cell counts during Ca-DTPA or Zn-DTPA therapy. (2.4, 5.2)
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dose
Administer Ca-DTPA as the initial dose during the first 24 hours after internal contamination. Ca-DTPA is more effective than Zn-DTPA during this time period (see Ca-DTPA labeling). If Ca-DTPA is not available, use Zn-DTPA as initial therapy. On the next day, if additional chelation therapy is indicated, begin daily treatment with Zn-DTPA. If Zn-DTPA is not available, chelation therapy may continue with Ca-DTPA and concomitant mineral supplements containing zinc should be given (see Ca-DTPA labeling).
Do not administer more than one dose per 24 hour period.
If Ca-DTPA is not available during the first 24 hours:
- in adults and adolescents, administer intravenously a single 1.0 gram initial dose of Zn-DTPA.
- in children less than 12 years of age, administer intravenously a single 14 mg/kg initial dose of Zn-DTPA, not to exceed 1.0 gram.
After the first 24 hours, continue chelation therapy with Zn-DTPA:
- in adults and adolescents, administer intravenously 1.0 gram Zn-DTPA once daily.
- in children less than 12 years of age, administer intravenously 14 mg/kg Zn-DTPA once daily, not to exceed 1.0 gram daily.
Renally Impaired Patients
No dose adjustment is needed. However, renal impairment may reduce the rate at which chelators remove radiocontaminants from the body. In heavily contaminated patients with renal impairment, dialysis may be used to increase the rate of elimination. High efficiency high flux dialysis is recommended. Because dialysis fluid will become radioactive, radiation precautions must be taken to protect personnel, other patients, and the general public.
2.2 General
Chelation treatment is most effective if administered within the first 24 hours after internal contamination. Start chelation treatment as soon as possible after suspected or known internal contamination. When treatment cannot be started right away, give chelation treatment as soon as it becomes available. Chelation treatment is still effective even after time has elapsed following internal contamination. The chelating effects of Zn-DTPA are greatest when the radiocontaminants are still circulating or are in interstitial fluids. The effectiveness of chelation decreases with time following internal contamination as the radiocontaminants become sequestered in liver and bone.
If internal contamination with radiocontaminants other than plutonium, americium, or curium, or unknown radiocontaminants is suspected, additional therapies may be needed (e.g., Prussian blue, potassium iodide).
2.3 Methods of Administration
Use intravenous administration of Zn-DTPA if the route of internal contamination is not known or if multiple routes of internal contamination are likely. Administer Zn-DTPA solution (1 gram in 5 mL) either with a slow intravenous push over a period of 3-4 minutes or by intravenous infusion over 30 minutes diluted in 100-250 mL of 5% dextrose in water (D5W), Ringers Lactate, or Normal Saline.
In individuals whose internal contamination is only by inhalation, Zn-DTPA can be administered by nebulized inhalation as an alternative route of administration.
Dilute Zn-DTPA for nebulization at a 1:1 ratio with sterile water or saline. After nebulization, encourage patients to avoid swallowing any expectorant. Some individuals may experience respiratory adverse events after inhalation therapy. [See Warnings and Precautions (5.1)] The safety and effectiveness of the nebulized route of administration have not been established in the pediatric population.
The safety and effectiveness of the intramuscular route of injection have not been established.
2.4 Monitoring
When possible, obtain baseline blood and urine samples (CBC with differential, BUN, serum chemistries and electrolytes, urinalysis and blood and urine radioassays) before initiating treatment.
To establish an elimination curve, obtain a quantitative baseline estimate of the total internalized transuranium element(s) and measures of elimination of radioactivity by appropriate whole-body counting, by bioassay (e.g., biodosimetry), or fecal/urine sample whenever possible.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Asthma Exacerbation
Nebulized chelation therapy is associated with asthma exacerbation. Monitor patients for signs and symptoms of asthma exacerbation when administering Zn-DTPA by the inhalation route. [See Adverse Reactions (6)]
5.2 Depletion of Body Trace Mineral Stores
Zn-DTPA treatment may lead to depletion of body stores of endogenous metals (e.g. magnesium, manganese). The risk for depletion increases when Zn-DTPA is administered over several months. Monitor serum zinc levels, electrolytes and blood cell counts during Ca-DTPA or Zn-DTPA therapy. Give mineral or vitamin plus mineral supplements as appropriate. [See Dosage and Administration (2.4)]
5.3 Risks to Care-takers
Radioactive metals are known to be excreted in the urine, feces, and breast milk. In individuals with recent internal contamination with plutonium, americium, or curium, Zn-DTPA treatment increases excretion of radioactivity in the urine. Take appropriate safety measures to minimize contamination of others. [See Patient Counseling Information (17)]
-
6 ADVERSE REACTIONS
In the U.S. Registry, a total of 646 individuals received at least one dose of either Ca-DTPA or Zn-DTPA. Of these, 62 received Zn-DTPA by one or more routes of administration. Forty-eight individuals were dosed by intravenous administration, 18 by inhalation and 8 by other or unknown routes of administration.
Of the individuals that received Zn-DTPA, 23/62 (37%) received one dose and 8 (13%) received two doses. The remaining 31 individuals received three or more doses. The largest number of Zn-DTPA doses to a single individual was 574 doses delivered over 3.5 years.
Overall, the presence or absence of adverse events was recorded in 310/646 individuals. Of these 19 (6.1%) individuals reported at least one adverse event. The total number of recorded adverse events was 20. Of the 20 adverse events, 1 individual treated with Zn-DTPA reported headache, lightheadedness, and pelvic pain.
Two individuals experienced cough and/or wheezing with nebulized Ca-DTPA therapy however there was no report of such events with nebulized Zn-DTPA.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Risk Summary
There are no adequate and well-controlled studies of Zn-DTPA use in pregnant women. Chelation treatment of pregnant women should begin and continue with Zn-DTPA. Reproduction studies have been performed in pregnant mice at doses up to 31 times (11.5 mmol/kg) the recommended daily human dose and have revealed no evidence of impaired fertility or harm to the fetus due to Zn-DTPA. There was a slight reduction in the average birth weight. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
8.3 Nursing mothers
It is not known whether Zn-DTPA is excreted in human milk. Radiocontaminants are known to be excreted in breast milk. Women with known or suspected internal contamination with radiocontaminants should not breast feed, whether or not they are receiving chelation therapy. Precautions should be taken when discarding breast milk. [See Warnings and Precautions (5.3)]
8.4 Pediatric use
The safety and effectiveness of Zn-DTPA were established in the adult population and efficacy was extrapolated to the pediatric population for the intravenous route based on the comparability of pathophysiologic mechanisms. The dose is based on body size adjustment for an intravenous drug that is renally cleared [See Dosage and Administration (2.1)]. The safety and effectiveness of the nebulized route of administration have not been established in the pediatric population.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Pentetate zinc trisodium injection contains the sodium salt of zinc diethylenetriaminepentaacetate. Pentetate zinc trisodium is also known as trisodium zinc diethylenetriaminepentaacetate and is commonly referred to as Zn-DTPA. It has a molecular formula of Na3ZnC14H18N3O10 and a molecular weight of 522.7 Daltons. It is represented by the following structural formula:
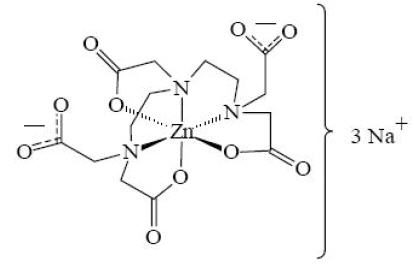
Zn-DTPA is supplied as a clear, colorless, hyperosmolar (1260 mOsmol/kg) solution in a colorless ampoule containing 5 mL. The ampoule contents are sterile, non-pyrogenic and suitable for intravenous administration. Each mL of solution contains the equivalent of 200 mg pentetate zinc trisodium (obtained from 150.51 mg pentetic acid, 31.14 mg zinc oxide and NaOH) and water for injection, USP. The pH of the solution is adjusted with NaOH and is between 6.5-7.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Zn-DTPA forms stable chelates with metal ions by exchanging zinc for a metal of greater binding capacity. The radioactive chelates are then excreted by glomerular filtration into the urine. In animal studies, Zn-DTPA forms less stable chelates with uranium and neptunium in vivo resulting in deposition of these elements in tissues including the bone. Zn-DTPA treatments are not expected to be effective for uranium and neptunium. Radioactive iodine is not bound by DTPA.
12.2 Pharmacodynamics
In a study of rodents internally contaminated with plutonium, the rate of plutonium elimination was measured after treatment with Ca-DTPA and Zn-DTPA given intravenously as a single dose of 10 to 1,000 micromol/kg (0.54-54 × maximum human dose, MHD). When treated within one hour of internal contamination, Ca-DTPA resulted in about a 10 fold higher rate of elimination of plutonium in the urine as compared to Zn-DTPA. The chelating capacity of Ca-DTPA is greatest immediately and up to approximately 24 hours after internal contamination when the radiocontaminant is still circulating and readily available for chelation. After the first dose of Ca-DTPA, maintenance treatment with either Ca-DTPA or Zn-DTPA resulted in similar rates of elimination of radioactivity. However, at comparable doses, Zn-DTPA had less toxicity (e.g., less depletion of trace metals, lower rate of mortality, the absence of kidney and liver vacuolization, and absence of small bowel hemorrhagic lesions).
In another study, rodents contaminated with aerosolized plutonium and americium were treated with Ca-DTPA and Zn-DTPA. The treatment schedule involved inhalation of Ca-DTPA 2 micromol/kg (0.11 MHD) 30 minutes after contamination followed by inhalation of Zn-DTPA 2 micromol/kg at approximately 6 hours, 1, 2, 3, and 6 days, then twice weekly to day 26 or day 27. The treatment regime reduced the lung deposit of plutonium and americium to 1-2% of that in untreated animals. Systemic deposit in liver and skeleton were reduced by half.
Literature and U.S. Registry data in humans indicate that intravenous administration of Zn-DTPA forms chelates with radioactive contaminants found in the circulation, interstitial fluid, and tissues. When Zn-DTPA is administered by inhalation, it can chelate transuranium elements. Expectoration is expected to decrease the amount of radioactive contaminant available for systemic absorption.
The effectiveness of chelation decreases with time after internal contamination because the transuranium elements become incorporated into the tissues. Give chelation treatment as soon as possible after known or suspected internal contamination with transuranium elements has occurred. [See Dosage and Administration (2.1, 2.2)]
12.3 Pharmacokinetics
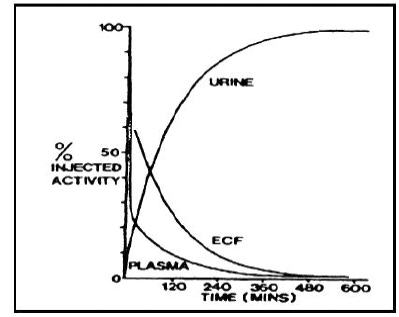
Plasma retention and urinary excretion data were obtained in 2 subjects that received 750 kBq of 14C-DTPA. As shown in Figure 1, the radiolabeled DTPA was rapidly distributed throughout the extracellular fluid space and was cleared by glomerular filtration. The plasma retention up to 7 hours post dosing was expressed by the sum of three exponential components with average half lives of 1.4 min, 14.5 min, and 94.4 min. The level of activity in the plasma was below the limit of detection 24 hours after injection. During the study, no detectable activity was exhaled or excreted in the feces. By 24 hours, cumulative urinary excretion was more than 99% of the injected dose.
Figure 1: Percent of 14C-DTPA Distribution
Absorption
Zn-DTPA is poorly absorbed in the gastrointestinal tract. In animal studies, after oral administration, absorption was approximately 5%. In a U.S. Registry of 18 patients who received a single inhaled or intravenous dose of 1 gram, urine data indicate that the inhaled product was absorbed and resulted in a comparable elimination of the radiocontaminant. One study of 2 human subjects that received Ca-DTPA with 14C-DTPA by inhalation revealed approximately 20% absorption from the lungs. Human or animal bioavailability comparisons for Zn-DTPA are not available after administration by inhalation and intravenous injection. [See Clinical Studies (14)]
Distribution
Following intravenous administration, Zn-DTPA is rapidly distributed throughout the extracellular fluid space. No significant amount of Zn-DTPA penetrates into erythrocytes or other cells. No accumulation of Zn-DTPA in specific organs has been observed. There is little or no binding of the chelating agent by the renal parenchyma.
Elimination
Zn-DTPA is cleared from the plasma in the first few hours after dosing through urinary excretion by glomerular filtration. Renal tubular excretion has not been documented. In stool samples, only a very small amount of radioactivity (<3%) was detected.
Renal Impaired and/or Compromised Liver Function Patients
Adequate and well-controlled pharmacokinetic and pharmacodynamic studies in renally impaired and/or hepatically impaired patients were not identified in the literature. Both Zn-DTPA and its radioactive chelates are excreted by glomerular filtration. Impaired renal function may decrease their rates of elimination and increase the serum half-life of Zn-DTPA.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
All clinical data has come from the treatment of individuals who were accidentally contaminated. Observational data were maintained in a U.S. Registry of individuals with internal radiation contamination primarily from acute occupational contamination with plutonium, americium and curium.
In 286 individuals, bioassays were available to measure urinary radioactivity elimination after chelation therapy. Of these 286 individuals, 18 had matched pre- and post-chelator urine radioactivity bioassay results available. The majority of these individuals received Ca-DTPA as the initial component to their chelation therapy. When multiple chelator doses were administered over days, the standard of practice was to switch therapy to Zn-DTPA following an initial dose of Ca-DTPA. Although both chelators were considered equipotent 24 hours following internal contamination, Zn-DTPA was considered less toxic. In one individual who received 3 doses, 1 gram each, by nebulization (1:1 Zn-DTPA and saline) followed by 6 intravenous doses, the urinary excretion of plutonium after the first nebulized dose was increased by a factor of 45.
After initial treatment with Ca-DTPA, maintenance treatment was continued with daily 1 gram Zn-DTPA doses administered over a period of days, months or years, depending on the extent of internal contamination and individual response to therapy. Treatment was generally continued until the excretion enhancement factor (EEF) approached 1. The longest treatment duration was 3.5 years. Similar increases in urinary radioactivity elimination were supported by data from the remaining 268 individuals in the U.S. Registry and from the literature.
-
16 HOW SUPPLIED
Zn-DTPA is supplied as a sterile solution in 5 mL single-use clear glass ampoules at a concentration of 200 mg/mL for intravenous use. Each ampoule contains the equivalent of 1000 mg of pentetate zinc trisodium.
NDC: 70651-002-03: 5 mL single-dose ampoules, package of 10
16.3 Handling
Inspect parenteral drug products visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The product may be filtered using a sterile filter if particles are seen subsequent to opening of the ampoule.
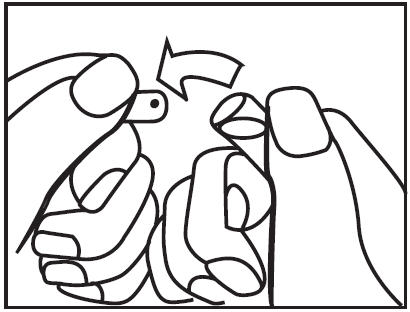
OPC ampoule: to open, turn so that the point faces upward and break off the neck with a downward movement.
-
17 PATIENT COUNSELING INFORMATION
Instruct patients to:
- drink plenty of fluids and void frequently to promote dilution of the radioactive chelate in the urine and minimize radiation exposure directly to the bladder.
- use a toilet instead of a urinal, and flush several times after each use.
- clean up spilled urine or feces completely and wash hands thoroughly. Wash clothing or linens separately if blood or urine comes in contact.
- dispose of any expectorant carefully. Avoid swallowing the expectorant if possible.
IInstruct parents and child-care givers to take extra precaution in handling the urine, feces, and expectorants of children to avoid any additional exposure to either the care-giver or to the child.
Instruct nursing mothers to take extra precaution in disposing of breast milk. [See Use in Specific Populations (8.3)]
-
18 COLLECTION OF PATIENT TREATMENT DATA
To develop long-term response data and information on the risk of developing late malignancy, detailed information on patient treatment should be provided to the manufacturer (see Patient Treatment Data Form). To obtain additional forms, please use the enclosed form as a template or see the following website: www.zn-dtpa.com. These data should include a record of the radioactive body burden and bioassay results at defined time intervals, a description of measurement methods to facilitate analysis of data, and adverse events.
Questions regarding the use of Zn-DTPA for the treatment of internal contamination with transuranium elements may be referred to:
hameln pharmaceuticals ltd
Nexus
Gloucester Business Park
Gloucester, GL3 4AG
United Kingdom
Tel: + 44 / 1452 / 621661
Fax: +44 / 1452 / 632732
e-mail: drugsafety@hameln.co.ukContact person: Richard Wysocki
Phone: +44 / 1452 / 621661
Fax: +44 / 1452 / 632732
Email: r.wysocki@hameln.co.ukRevised: 10/2016
44640/17/16
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 5 mL Ampoule Package
-
INGREDIENTS AND APPEARANCE
PENTETATE ZINC TRISODIUM
pentetate zinc trisodium injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70651-002 Route of Administration INTRAVENOUS, RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Pentetate zinc trisodium (UNII: NXU65IC8PG) (pentetic acid - UNII:7A314HQM0I) Pentetate zinc trisodium 1000 mg in 5 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Zinc oxide (UNII: SOI2LOH54Z) sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70651-002-03 10 in 1 PACKAGE 08/11/2004 1 5 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021751 08/11/2004 Labeler - hameln pharma plus gmbh (319361341) Establishment Name Address ID/FEI Business Operations Siegfried Hameln Gmbh 315869123 MANUFACTURE(70651-002) , ANALYSIS(70651-002) , LABEL(70651-002) , PACK(70651-002)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.