MICROBALANCE- lactobacillus acidophilus and bifidobacterium lactis capsule
MICROBALANCE by
Drug Labeling and Warnings
MICROBALANCE by is a Other medication manufactured, distributed, or labeled by Blue Heron Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- HEALTH CLAIM:
-
DESCRIPTION:
Microbalance Probiotic Capsules are clear capsules dispensed in plastic bottles of 30 ct.
85622-0102-30
Reserved for Professional Recommendation
Microbalance Probiotic Capsules should be administered under the supervision of a licensed medical practitioner.
Microbalance is an orally administered prescription probiotic formulation for the clinical dietary management of suboptimal nutritional status in patients where advanced supplementation is required and nutritional supplementation in physiologically stressful conditions for maintenance of good health is needed.
This listed product is not a National Drug Code, but instead has merely been formatted to comply with standard industry practice for pharmacy and insurance computer systems.
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. This product may be administered only under a physician’s supervision. There are no implied or explicit claims on therapeutic equivalence.
Manufactured for:
Blue Heron Pharmaceuticals
Lutz, FL 33559
-
WARNINGS:
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
As with any supplement, if you are pregnant, nursing, taking medication, or have a medical condition, ask a health professional before taking this product.
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
- DOSAGE AND ADMINISTRATION:
-
PRECAUTIONS:
CONTRAINDICATIONS
This product is contraindicated in patients with known hypersensitivity to any of the ingredients.PRECAUTIONS
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.ADVERSE REACTIONS
Allergic sensitizations have been reported following oral administration of folic acid. Consult your physician immediately if adverse side effects occur.KEEP OUT OF THE REACH OF CHILDREN.
-
STORAGE:
STORAGE:
Handling (before opening):
Product is sensitive to moisture and heat. Keep bottle tightly closed in a dry, cool place. Store at 200C to 250C (680F to 770F).Handling (after opening):
Store in the refrigerator after opening, DO NOT FREEZE. Store at 20C to 80C (350F to 460F). - PACKAGING:
-
INGREDIENTS AND APPEARANCE
MICROBALANCE
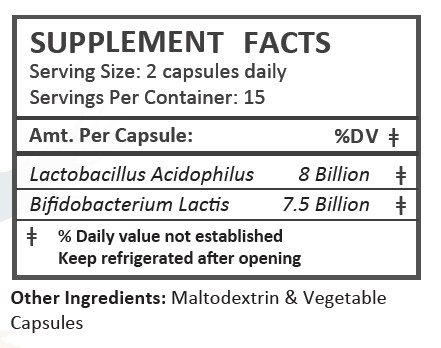
lactobacillus acidophilus and bifidobacterium lactis capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:85622-102 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LACTOBACILLUS ACIDOPHILUS (UNII: 1PRR1V42V5) (LACTOBACILLUS ACIDOPHILUS - UNII:1PRR1V42V5) LACTOBACILLUS ACIDOPHILUS 8000000000 [CFU] BIFIDOBACTERIUM ANIMALIS LACTIS (UNII: 5307V7XW8I) (BIFIDOBACTERIUM ANIMALIS LACTIS - UNII:5307V7XW8I) BIFIDOBACTERIUM ANIMALIS LACTIS 7500000000 [CFU] Inactive Ingredients Ingredient Name Strength MALTODEXTRIN (UNII: 7CVR7L4A2D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:85622-102-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 09/19/2025 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 25 mm scoring 1 Labeler - Blue Heron Pharmaceuticals, LLC (137019051)
Trademark Results [MICROBALANCE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MICROBALANCE 87858694 5606473 Live/Registered |
Shenzhen Wanxin Biointelligent Technology Co.,Ltd. 2018-04-02 |
 MICROBALANCE 86195461 not registered Dead/Abandoned |
Hennessy Industries, Inc. 2014-02-17 |
 MICROBALANCE 79107361 4188663 Live/Registered |
Nutreco IP Assets B.V. 2011-09-21 |
 MICROBALANCE 77905794 3895287 Dead/Cancelled |
PRIDE MOBILITY PRODUCTS CORPORATION 2010-01-06 |
 MICROBALANCE 76042082 2682230 Dead/Cancelled |
RATH, MATTHIAS 2000-05-05 |
 MICROBALANCE 75444677 not registered Dead/Abandoned |
ADVANCED POLYMER SYSTEMS, INC. 1998-03-04 |
 MICROBALANCE 75044418 not registered Dead/Abandoned |
ADVANCED POLYMER SYSTEMS, INC. 1996-01-16 |
 MICROBALANCE 74149582 1680525 Dead/Cancelled |
New Generation Foods, Inc. 1991-03-20 |
 MICROBALANCE 73535329 1373372 Dead/Cancelled |
NEW GENERATION FOODS, INC. 1985-05-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.