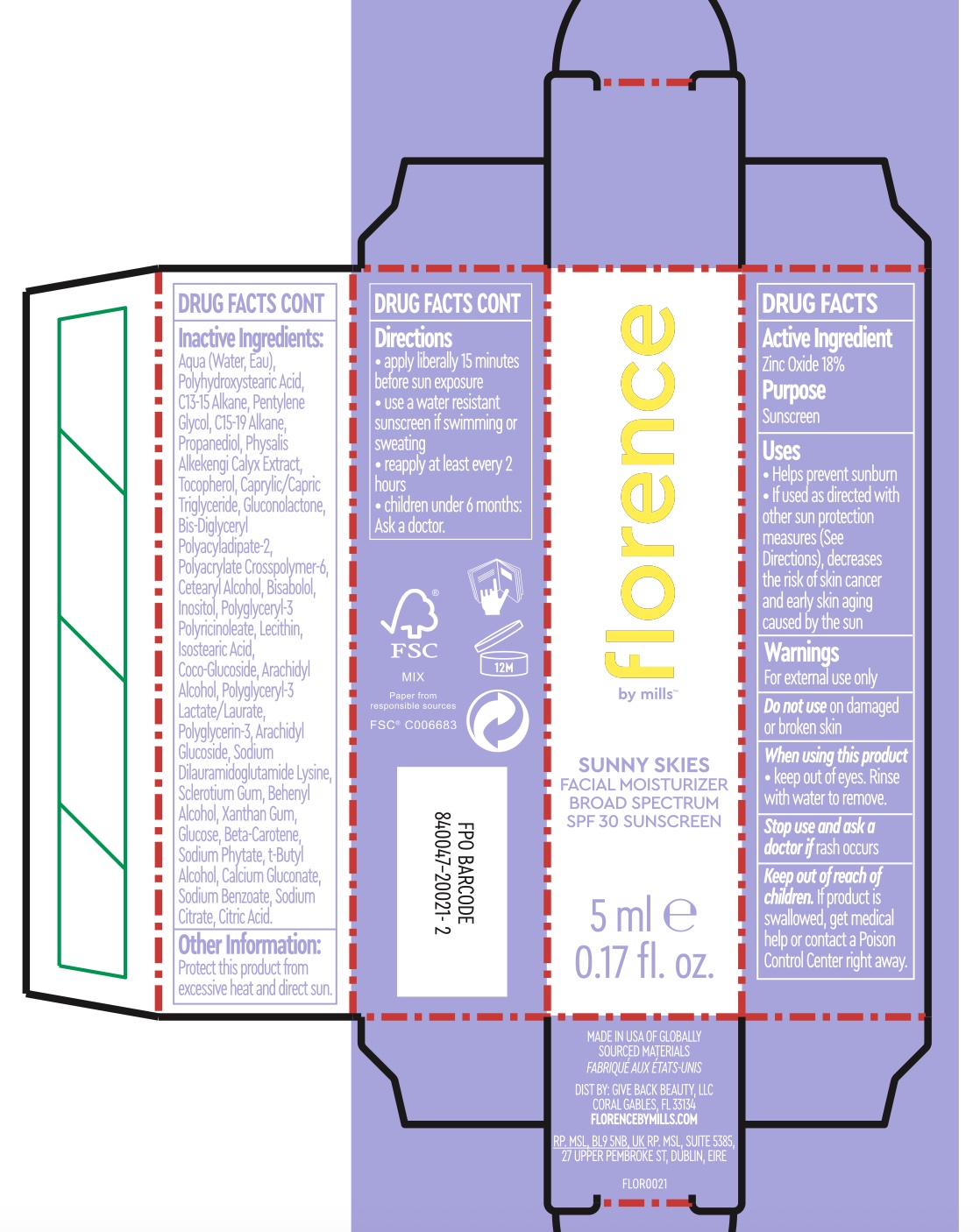
Sunny Skies Facial Moisturizer, SPF 30 - 85623-101
Sunny Skies Facial Moisturizer by
Drug Labeling and Warnings
Sunny Skies Facial Moisturizer by is a Otc medication manufactured, distributed, or labeled by Give Back Beauty, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SUNNY SKIES FACIAL MOISTURIZER- zinc oxide lotion
Give Back Beauty, LLC
----------
Sunny Skies Facial Moisturizer, SPF 30 - 85623-101
Uses
Helps prevent sunburn
If used as directed with other sun protection measures (See
Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only
Do not use on damaged or broken skin
When using this product
keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Inactive Ingredients
Aqua (Water, Eau), Polyhydroxystearic Acid, C13-15 Alkane, Pentylene Glycol, C15-19 Alkane, Propanediol, Physalis Alkekengi Calyx Extract, Tocopherol, Caprylic/Capric Triglyceride, Gluconolactone, Bis-Diglyceryl Polyacyladipate-2, Polyacrylate Crosspolymer-6, Cetearyl Alcohol, Bisabolol, Inosito, Polyglycery-3. Polyricinoleate, Lecithin, Isostearic Acid, Coco-Glucoside, Arachidyl Alcohol, Polyglycer -3 Lactate/Laurate, Polyglycerin-3, Arachidyl Glucoside, Sodium Dilauramidoglutamide Lysine, Sclerotium Gum, Behenyl Alcohol, Xanthan Gum, Glucose, Beta-Carotene, Sodium Phytate, t-Butyl Alcohol, Calcium Gluconate, Sodium Benzoate, Sodium Citrate, Citric Acid.
| SUNNY SKIES FACIAL MOISTURIZER
zinc oxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Give Back Beauty, LLC (117297657) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.